Biomembranes Contain Three Principal Classes of Lipids
The term phospholipid is a somewhat generic term. It refers to any amphipathic lipid with a phosphate-based head group and a two-chain hydrophobic tail. A typical biomembrane is not composed of phospholipids alone, but actually contains three classes of amphipathic lipids: phosphoglycerides, sphingolipids, and sterols, which differ in their chemical structures, abundance, and functions in the membrane (Figure 7-8). While all phosphoglycerides are phospholipids, only certain sphingolipids are, and no sterols are.
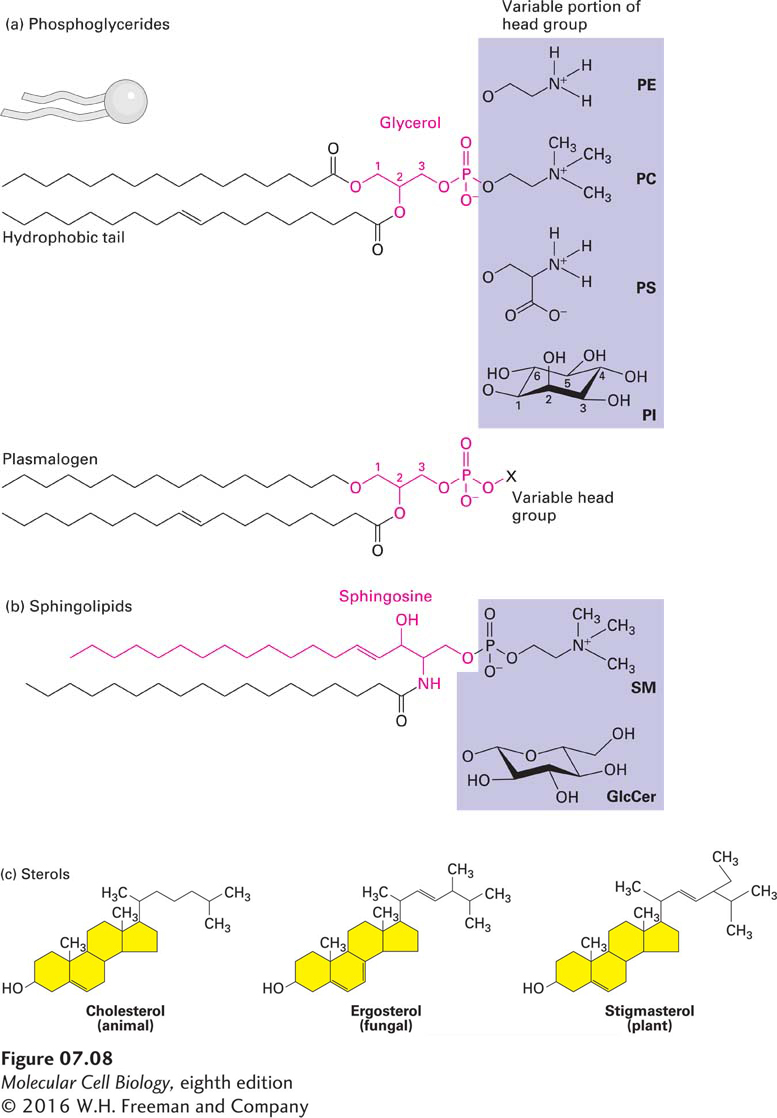
FIGURE 7-8 Three classes of membrane lipids. (a) Most phosphoglycerides are derivatives of glycerol 3-phosphate (red), which contains two esterified fatty acyl chains that constitute the hydrophobic “tail” and a polar “head group” esterified to the phosphate. The fatty acids can vary in length and be saturated (no double bonds) or unsaturated (one, two, or three double bonds). In phosphatidylcholine (PC), the head group is choline. Also shown are the molecules attached to the phosphate group in three other common phosphoglycerides: phosphatidylethanolamine (PE), phosphatidylserine (PS), and phosphatidylinositol (PI). Plasmalogens contain one fatty acyl chain attached to glycerol by an ester linkage and one attached by an ether linkage; they contain the same head groups as other phosphoglycerides. (b) Sphingolipids are derivatives of sphingosine (red), an amino alcohol with a long hydrocarbon chain. Various fatty acyl chains are connected to sphingosine by an amide bond. The sphingomyelins (SM), which contain a phosphocholine head group, are phospholipids. Other sphingolipids are glycolipids in which a single sugar residue or branched oligosaccharide is attached to the sphingosine backbone. For instance, the simple glycolipid glucosylcerebroside (GlcCer) has a glucose head group. (c) The major sterols in animals (cholesterol), fungi (ergosterol), and plants (stigmasterol) differ slightly in structure, but all serve as key components of cellular membranes. The basic structure of sterols is a four-ring hydrocarbon (yellow). Like other membrane lipids, sterols are amphipathic. The single hydroxyl group is equivalent to the polar head group in other lipids; the conjugated ring and short hydrocarbon chain form the hydrophobic tail. See H. Sprong et al., 2001, Nature Rev. Mol. Cell Biol. 2:504.
Phosphoglycerides, the most abundant class of phospholipids in most membranes, are derivatives of glycerol 3-phosphate (see Figure 7-8a). A typical phosphoglyceride molecule consists of a hydrophobic tail composed of two fatty acid–based (acyl) chains esterified to the two hydroxyl groups in glycerol phosphate and a polar head group attached to the phosphate group. The structure comprising the glycerol moiety and the two fatty acyl chains is referred to as a diacylglycerol. The two fatty acyl chains may differ in the number of carbons that they contain (commonly 16 or 18) and their degree of saturation (0, 1, or 2 double bonds). A phosphoglyceride is classified according to the nature of its head group. In phosphatidylcholines, the most abundant phospholipids in the plasma membrane, the head group consists of choline, a positively charged alcohol, esterified to the negatively charged phosphate. In other phosphoglycerides, an OH-containing molecule such as ethanolamine, serine, or the sugar derivative inositol is linked to the phosphate group. In the case of the inositol head group, the hydroxyl groups may be further modified with phosphates, yielding a class of phospholipids called phosphoinositides. The phosphoinositides fulfill an important function in signal transduction. The negatively charged phosphate group and the positively charged groups or hydroxyl groups on the head group interact strongly with water. At neutral pH, some phosphoglycerides (e.g., phosphatidylcholine and phosphatidylethanolamine) carry no net electric charge, whereas others (e.g., phosphatidylinositol and phosphatidylserine) carry a single net negative charge. Nonetheless, the polar head groups in all these phospholipids can pack together into the characteristic bilayer structure. When phospholipases act on phosphoglycerides, they produce lysophospholipids, which lack one of the two acyl chains. Lysophospholipids are not only important signaling molecules, released from cells and recognized by specific receptors; their presence can also affect the physical properties of the membranes in which they reside.
The plasmalogens are a group of phosphoglycerides that contain one fatty acyl chain attached to carbon 2 of glycerol by an ester linkage and one long hydrocarbon chain attached to carbon 1 of glycerol by an ether (C–O–C) rather than an ester linkage. Plasmalogens are particularly abundant in human brain and heart tissue. The greater chemical stability of the ether linkage in plasmalogens compared with the ester linkage, and the subtle differences in three-dimensional structure between plasmalogens and other phosphoglycerides, may have as yet unrecognized physiological significance.
A second class of membrane lipids is the sphingolipids. All these compounds are derived from sphingosine, an amino alcohol with a long hydrocarbon chain, and contain a long-chain fatty acid attached in amide linkage to the sphingosine amino group (see Figure 7-8b). Like phosphoglycerides, some sphingolipids have a phosphate-based polar head group. In sphingomyelin, the most abundant sphingolipid, phosphocholine is attached to the terminal hydroxyl group of sphingosine (see Figure 7-8b, SM). Thus sphingomyelin is a phospholipid, and its overall structure is quite similar to that of phosphatidylcholine. Sphingomyelins are similar in shape to phosphoglycerides and can form mixed bilayers with them. Other sphingolipids are amphipathic glycolipids whose polar head groups are sugars that are not linked to the tails via a phosphate group (so they are technically not phospholipids). Glucosylcerebroside, the simplest glycosphingolipid, contains a single glucose unit attached to sphingosine. In the complex glycosphingolipids called gangliosides, one or two branched sugar chains (oligosaccharides) containing sialic acid groups are attached to sphingosine. Glycolipids constitute 2–10 percent of the total lipid content of plasma membranes; they are most abundant in nervous tissue.
Cholesterol and its analogs constitute the third important class of membrane lipids, the sterols. The basic structure of sterols is a four-ring isoprenoid-based hydrocarbon. The structures of the principal yeast sterol (ergosterol) and plant phytosterols (e.g., stigmasterol) differ slightly from that of cholesterol, the major animal sterol (see Figure 7-8c). The small differences in the biosynthetic pathways and structures of fungal and animal sterols are the basis of most antifungal drugs currently in use. Cholesterol, like the two other sterols, has a hydroxyl substituent on one ring. Although cholesterol is almost entirely hydrocarbon in composition, it is amphipathic because its hydroxyl group can interact with water. Because it lacks a phosphate-based head group, it is not a phospholipid. Cholesterol is especially abundant in the plasma membranes of mammalian cells but is absent from most prokaryotic and all plant cells. As much as 30–50 percent of the lipids in plant plasma membranes consist of certain steroids unique to plants. Between 50 and 90 percent of the cholesterol in most mammalian cells is present in the plasma membrane and associated vesicles. Cholesterol and other sterols are too hydrophobic to form a bilayer structure on their own. Instead, at the concentrations found in natural membranes, these sterols must intercalate between phospholipid molecules to be incorporated into biomembranes. When so intercalated, sterols provide structural support to membranes, preventing too close a packing of the phospholipids’ acyl chains to maintain a significant measure of membrane fluidity, and at the same time conferring the necessary rigidity required for mechanical support. Some of these effects can be highly local, as in the case of lipid rafts, discussed below.
In addition to its structural role in membranes, cholesterol is the precursor for several important bioactive molecules. They include bile acids, which are made in the liver and help emulsify dietary fats for digestion and absorption in the intestines; steroid hormones produced by endocrine cells (e.g., adrenal gland, ovary, testes); and vitamin D produced in the skin and kidneys. Another critical function of cholesterol is its covalent addition to Hedgehog protein, a key signaling molecule in embryonic development (see Chapter 16).
