Addition of Telomeric Sequences by Telomerase Prevents Shortening of Chromosomes
Sequencing of telomeres from multiple organisms, including humans, has shown that most are repetitive oligomers with a high G content located in the strand with its 3′ end at the end of the chromosome. The telomere repeat sequence in humans and other vertebrates is TTAGGG. These simple sequences are repeated at the very termini of chromosomes for a total of a few hundred base pairs in yeasts and protozoans and a few thousand base pairs in vertebrates. The 3′ end of the G-rich strand extends 12–16 nucleotides beyond the 5′ end of the complementary C-rich strand. This region is bound by specific proteins that protect the ends of linear chromosomes from attack by exonucleases.
The need for a specialized region at the ends of eukaryotic chromosomes is apparent when we consider that all known DNA polymerases elongate DNA chains at the 3′ end, and all require an RNA or DNA primer. As the replication fork approaches the end of a linear chromosome, synthesis of the leading strand continues to the end of the DNA template strand, completing one daughter DNA double helix. However, because the lagging-strand template is copied in a discontinuous fashion, it cannot be replicated in its entirety (Figure 8-43). When the final RNA primer is removed, there is no upstream strand onto which DNA polymerase can build to fill the resulting gap. Without some special mechanism, the daughter DNA strand resulting from lagging-strand synthesis would be shortened at each cell division.
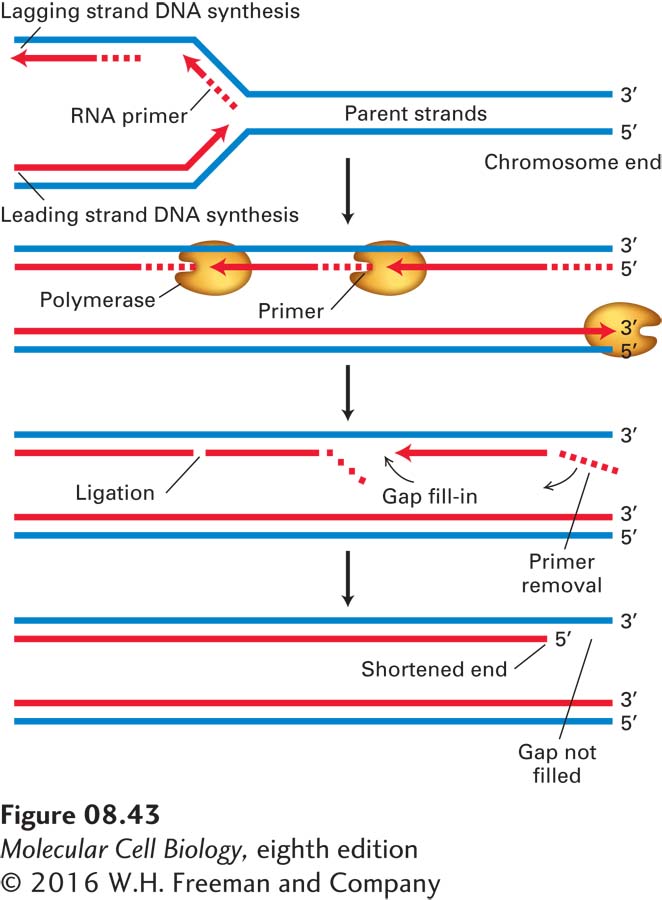
FIGURE 8-43 Standard DNA replication leads to loss of DNA at the 5′ end of each strand of a linear DNA molecule. Replication of the right end of a linear DNA is shown; the same process occurs at the left end (as can be shown by inverting the figure). As the replication fork approaches the end of the parental DNA molecule, the leading strand can be synthesized all the way to the end of the template strand without the loss of deoxyribonucleotides. However, since synthesis of the lagging strand requires RNA primers, the right end of the lagging daughter DNA strand would remain as ribonucleotides, which are removed and therefore cannot serve as the template for a replicative DNA polymerase. Alternative mechanisms must be used to prevent successive shortening of the lagging strand with each round of replication.
The problem of telomere shortening is solved by an enzyme that adds telomeric repeat sequences to the ends of each chromosome. The enzyme is a protein–RNA complex called telomere terminal transferase, or telomerase. Because the sequence of the telomerase-associated RNA, as we will see, serves as the template for addition of deoxyribonucleotides to the ends of telomeres, the source of the enzyme, and not the source of the telomeric DNA primer, determines the sequence added. This was proved by transforming Tetrahymena with a mutated form of the gene encoding the telomerase-associated RNA. The resulting telomerase added a DNA sequence complementary to the mutated RNA sequence to the ends of telomeric primers. Thus telomerase is a specialized form of a reverse transcriptase that carries its own internal RNA template to direct DNA synthesis. These experiments also earned the Nobel Prize in Physiology or Medicine for the structure and function of telomeres in 2009.
Figure 8-44 depicts how telomerase, by reverse transcription of its associated RNA, elongates the 3′ end of the single-stranded DNA at the end of the G-rich strand mentioned above. Cells from knockout mice that cannot produce the telomerase-associated RNA exhibit no telomerase activity, and their telomeres shorten successively with each cell generation. Such mice can breed and reproduce normally for three generations before the long telomere repeats become substantially eroded. Then, the absence of telomere DNA results in adverse effects, including fusion of chromosome termini and chromosome loss. By the fourth generation, the reproductive potential of these knockout mice declines, and they cannot produce offspring after the sixth generation.
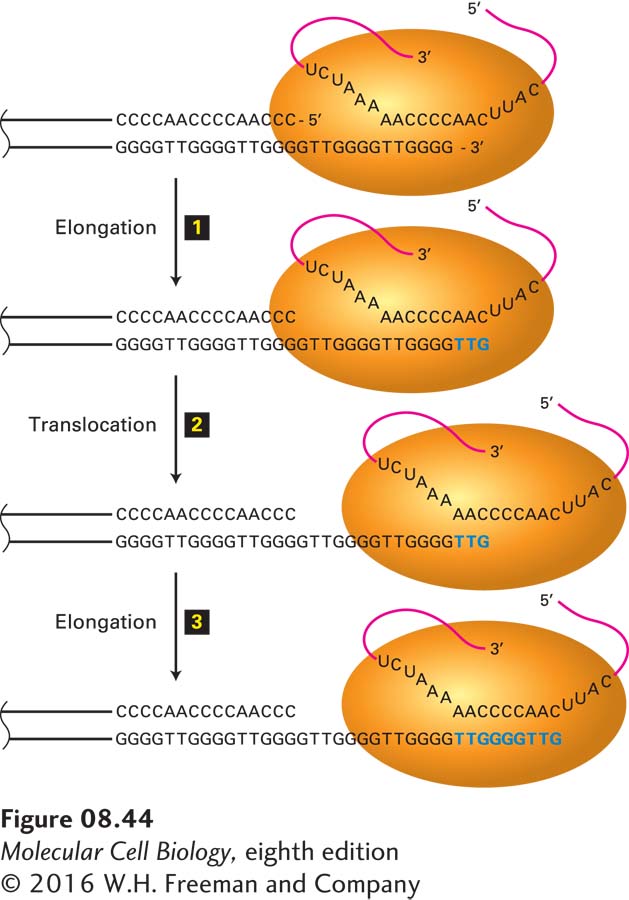
FIGURE 8-44 Mechanism of action of telomerase. The single-stranded 3′ terminus of a telomere is extended by telomerase, counteracting the inability of the DNA replication mechanism to synthesize the extreme terminus of linear DNA. Telomerase elongates this single-stranded end by a reiterative reverse-transcription mechanism. The action of the telomerase from the protozoan Tetrahymena, which adds a T2G4 repeat unit, is depicted here; other telomerases add slightly different sequences. The telomerase contains an RNA template (red) that base-pairs to the 3′ end of the lagging-strand template. The telomerase catalytic site then adds deoxyribonucleotides TTG (blue), using the RNA molecule as a template (step 1). The strands of the resulting DNA-RNA duplex are then thought to slip (translocate) relative to each other so that the TTG sequence at the 3′ end of the replicating DNA base-pairs to the complementary RNA sequence in the telomerase RNA (step 2). The 3′ end of the replicating DNA is then again extended by telomerase (step 3). Telomerases can add multiple repeats by repetition of steps 2 and 3. DNA polymerase α-primase can prime synthesis of new Okazaki fragments on this extended template strand. The net result prevents shortening of the lagging strand at each cycle of DNA replication. See C. W. Greider and E. H. Blackburn, 1989, Nature 337:331.
The human genes expressing the telomerase protein and the telomerase-associated RNA are active in germ cells and stem cells, but are turned off in most cells of adult tissues that replicate only a limited number of times, or will never replicate again (such cells are called postmitotic). However, these genes are activated in most human cancer cells, where telomerase is required for the multiple cell divisions necessary to form a tumor. This phenomenon has stimulated a search for inhibitors of human telomerase as potential therapeutic agents for treating cancer.
While telomerase prevents telomere shortening in most eukaryotes, some organisms use alternative strategies. Drosophila species maintain telomere lengths by the regulated insertion of non-LTR retrotransposons into telomeres. This is one of the few instances in which a mobile element has a specific function in its host organism.

