Initiation of lac Operon Transcription Can Be Repressed or Activated
When E. coli is in an environment that lacks lactose, synthesis of lac mRNA is repressed so that cellular energy is not wasted synthesizing enzymes the cell does not require. In an environment containing both lactose and glucose, E. coli cells preferentially metabolize glucose, the central molecule of carbohydrate metabolism. The cells metabolize lactose at a high rate only when lactose is present and glucose is largely depleted from the medium. They achieve this metabolic adjustment by repressing transcription of the lac operon until lactose is present and allowing synthesis of only low levels of lac mRNA until the cytosolic concentration of glucose falls to low levels. Transcription of the lac operon under different conditions is controlled by lac repressor protein and catabolite activator protein (CAP) (also called CRP, for cAMP receptor protein), each of which binds to a specific DNA sequence in the lac transcription-control region; these two sequences are called the operator and the CAP site, respectively (Figure 9-4, top).
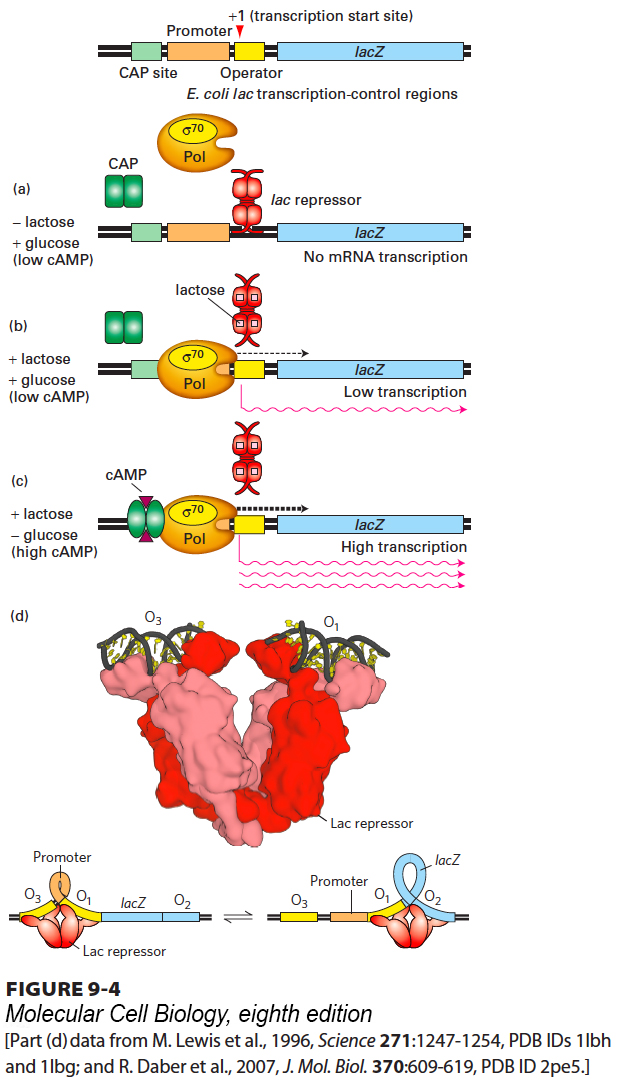
FIGURE 9-4 Regulation of transcription from the lac operon of E. coli. (Top) The transcription-control region, composed of roughly a hundred base pairs, includes three protein-binding regions: the CAP site, which binds catabolite activator protein; the lac promoter, which binds the σ70-RNA polymerase complex; and the lac operator, which binds lac repressor. The lacZ gene encoding the enzyme β-galactosidase, the first of the three genes in the operon, is shown to the right. (a) In the absence of lactose, very little lac mRNA is produced because the lac repressor binds to the operator, inhibiting transcription initiation by σ70-RNA polymerase. (b) In the presence of glucose and lactose, lac repressor binds lactose and dissociates from the operator, allowing σ70-RNA polymerase to initiate transcription at a low rate. (c) Maximal transcription of the lac operon occurs in the presence of lactose and the absence of glucose. In this situation, cAMP increases in response to the low glucose concentration and forms a CAP-cAMP complex, which binds to the CAP site, where it interacts with RNA polymerase to increase the rate of transcription initiation. (d) The tetrameric lac repressor binds to the primary lac operator (O1) and one of two secondary operators (O2 or O3) simultaneously. The two structures are in equilibrium. See B. Muller-Hill, 1998, Curr. Opin. Microbiol. 1:145.
[Part (d) data from M. Lewis et al., 1996, Science 271:1247-1254, PDB IDs 1lbh and 1lbg; and R. Daber et al., 2007, J. Mol. Biol. 370:609-619, PDB ID 2pe5.]
For transcription of the lac operon to begin, the σ70 subunit of the RNA polymerase must bind to the lac promoter at the −35 and −10 promoter sequences. When no lactose is present, the lac repressor binds to the lac operator, which overlaps the transcription start site. Therefore, the lac repressor bound to the operator site blocks σ70 binding and hence transcription initiation by RNA polymerase (Figure 9-4a). When lactose is present, it binds to specific binding sites in each subunit of the tetrameric lac repressor, causing a conformational change in the protein that makes it dissociate from the lac operator. As a result, the polymerase can bind to the promoter and initiate transcription of the lac operon. However, when glucose is also present, the frequency of transcription initiation is very low, resulting in the synthesis of only low levels of lac mRNA and thus of the proteins encoded by the lac operon (Figure 9-4b). The frequency of transcription initiation is low because the −35 and −10 sequences in the lac promoter differ from the ideal σ70-binding sequences shown previously.
Once glucose is depleted from the medium and the intracellular glucose concentration falls, E. coli cells respond by synthesizing cyclic AMP (cAMP). As the concentration of cAMP increases, it binds to a site in each subunit of the dimeric CAP protein, causing a conformational change that allows the protein to bind to the CAP site in the lac transcription-control region. The bound CAP-cAMP complex interacts with the polymerase bound to the promoter, greatly increasing the frequency of transcription initiation. This activation leads to synthesis of high levels of lac mRNA and subsequently of the enzymes encoded by the lac operon (Figure 9-4c).
In fact, the lac operon is more complex than depicted in the simplified model in Figure 9-4a–c. The tetrameric lac repressor actually binds to two DNA sequences simultaneously, one at the primary operator (lacO1), which overlaps the region of DNA bound by RNA polymerase at the promoter, and the other at one of two secondary operators centered at +412 (lacO2), within the lacZ protein-coding region, and −82 (lacO3) (Figure 9-4d). The lac repressor tetramer is a dimer of dimers. Each dimer binds to one operator (Figure 9-4d). Simultaneous binding of the tetrameric lac repressor to the primary lac operator and one of the two secondary operators is possible because DNA is quite flexible, as we saw in the wrapping of DNA around the surface of a histone octamer in the nucleosomes of eukaryotes (see Figure 8-24). The secondary operators function to increase the local concentration of lac repressor in the micro-vicinity of the primary operator where repressor binding blocks RNA polymerase binding. Since the equilibrium of binding reactions depends on the concentrations of the binding partners, the resulting increased local concentration of lac repressor in the vicinity of O1 increases repressor binding to O1. There are approximately 10 lac repressor tetramers per E. coli cell. Because of binding to O2 and O3, there is nearly always a lac repressor tetramer much closer to O1 than would otherwise be the case if the 10 repressor tetramers were diffusing randomly through the cell. If both O2 and O3 are mutated so that the lac repressor no longer binds to them with high affinity, repression at the lac promoter is reduced by a factor of 70. Mutation of only O2 or only O3 reduces repression twofold, indicating that either one of these secondary operators can provide most of the increase in repression.
Although the promoters for different E. coli genes exhibit considerable homology, their exact sequences differ. The promoter sequence determines the intrinsic frequency at which RNA polymerase–σ complexes initiate transcription of a gene in the absence of a repressor or activator protein. Promoters that support a high frequency of transcription initiation have −10 and −35 sequences similar to the ideal promoter shown previously and are called strong promoters. Those that support a low frequency of transcription initiation differ from this ideal sequence and are called weak promoters. The lac operon, for instance, has a weak promoter whose sequence differs from the consensus strong promoter at several positions. Its low intrinsic frequency of initiation is further reduced by the lac repressor and substantially increased by the cAMP-CAP complex.
