The DNA sites to which nuclear receptors bind are called response elements. The characteristic nucleotide sequences of several response elements have been determined. The consensus sequences of response elements for two steroid hormone receptors, the glucocorticoid receptor response element (GRE) and the estrogen receptor response element (ERE) are 6-bp inverted repeats separated by any three base pairs (Figure 9-44a, b). This finding suggested that the cognate steroid hormone receptors would bind to DNA as symmetric dimers (i.e., dimers with twofold rotational symmetry), as was later confirmed by x-ray crystallographic analysis of the homodimeric glucocorticoid receptor’s C4 zinc-finger DNA-binding domain (see Figure 9-30b).
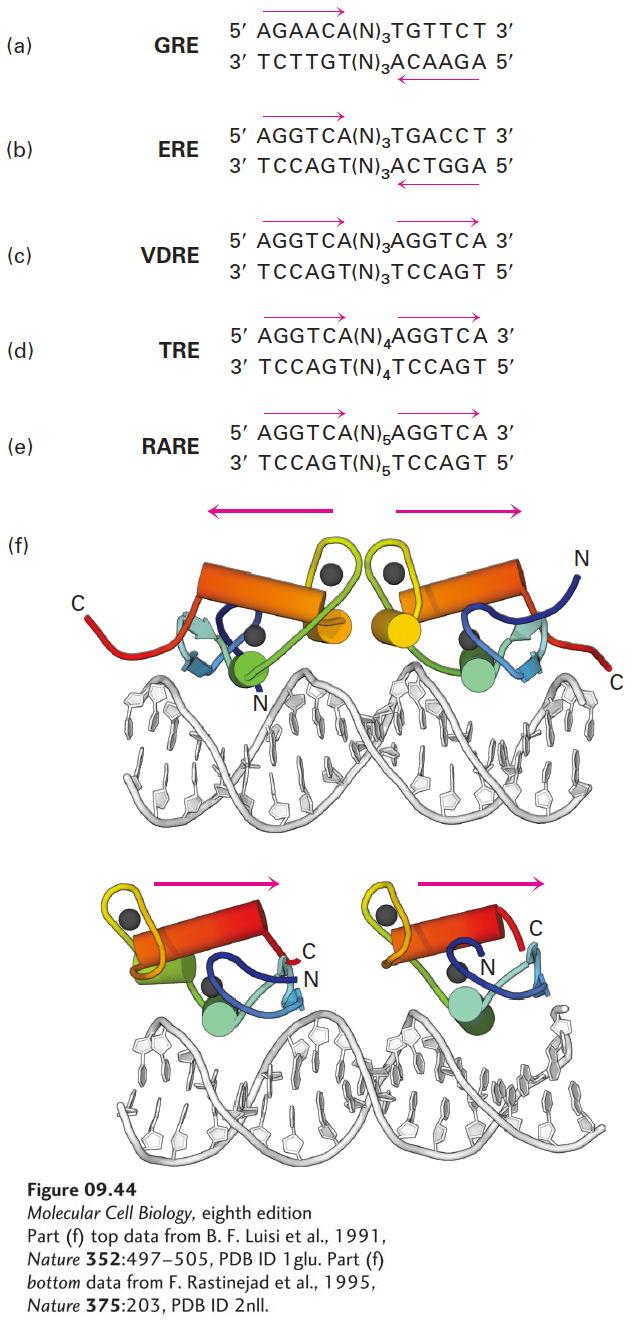
FIGURE 9-44 Consensus sequences of DNA response elements that bind five nuclear receptors. (a, b) The glucocorticoid and estrogen receptors are twofold symmetric dimers that bind, respectively, to the glucocorticoid receptor response element (GRE) and the estrogen receptor response element (ERE). Each of these response elements contains inverted repeats separated by three base pairs. (c–e) The heterodimeric nuclear receptors each contain one RXR subunit associated with another nuclear-receptor subunit that defines the hormone response. RXR-VDR mediates responses to vitamin D3 by binding to a direct repeat separated by three base pairs (a VDRE). RXR-TR mediates responses to thyroid hormone by binding to the same DNA bases in a direct repeat separated by four base pairs (a TRE). Similarly, RXR-RAR mediates a response to retinoic acid by binding to the same direct repeat separated by five base pairs, comprising a RARE. The repeat sequences bound by the reading helices of these receptors are indicated by red arrows. (f) Crystal structures of the glucocorticoid receptor bound to DNA containing a GRE (top) and of the RXR-TR heterodimer bound to DNA containing a TRE (bottom). Red arrows indicate the orientation from N to C of the helices below them. Note that in the twofold symmetric glucocorticoid receptor, the reading helices are inverted relative to each other so that they “read” an AGAACA on the top strand of the left half-site and on the bottom strand of the right half-site, separated by 3 base pairs. Consequently, the binding site for the glucocorticoid receptor and other twofold symmetric homodimers such as the estrogen receptor is an inverted repeat (see a and b). In contrast, the reading helices in the RXR-TR heterodimer are in the same orientation. Consequently, they read an AGGTCA sequence in the same orientation in the two-half sites separated by four base pairs, a direct-repeat binding site. The interface between the RXR subunit and the vitamin D3 receptor (VDR) subunit bound to a VDRE brings the two reading helices closer together so that they bind to the same half-sites separated by three rather than four base pairs. Similarly, the interface between the RXR and RAR subunits bound to a RARE positions the two reading helices in the heterodimer farther apart than in the RXR-TR, so that they bind the same AGGTCA sequences separated by five base pairs. See K. Umesono et al., 1991, Cell 65:1255, and A. M. Naar et al., 1991, Cell 65:1267.
[Part (f) top data from B. F. Luisi et al., 1991, Nature 352:497–505, PDB ID 1glu. Part (f) bottom data from F. Rastinejad et al., 1995, Nature 375:203, PDB ID 2nll.]
Some nuclear-receptor response elements, such as those for the receptors that bind nonsteroids such as vitamin D3, thyroid hormone, and retinoic acid, are direct repeats of the same sequence that is recognized by the estrogen receptor, separated by three, four, or five base pairs (Figure 9-44c–e). The specificity of these response elements is determined by the spacing between the repeats. The nuclear receptors that bind to these direct-repeat response elements do so as heterodimers, all of which share a monomer called RXR. The vitamin D3 response element (VDRE), for example, is bound by the RXR-VDR heterodimer, and the retinoic acid response element (RARE) is bound by RXR-RAR. The monomers composing these heterodimers interact with each other in such a way that the two DNA-binding domains lie in the same rather than inverted orientation, allowing the RXR heterodimers to bind to direct repeats of the binding site for each monomer (Figure 9-44f). In contrast, the monomers in homodimeric nuclear receptors (e.g., GRE and ERE) have an inverted orientation.
