Hormone Binding to a Nuclear Receptor Regulates Its Activity as a Transcription Factor
The mechanism whereby hormone binding controls the activity of nuclear receptors differs between heterodimeric and homodimeric receptors. Heterodimeric nuclear receptors (e.g., RXR-VDR, RXR-TR, and RXR-RAR) are located exclusively in the nucleus. In the absence of their hormone ligand, they repress transcription when bound to their cognate sites in DNA. They do so by directing histone deacetylation at nearby nucleosomes by associating with histone deacetylase complexes, as described earlier for other repressors (see Figure 9-37a). When heterodimeric nuclear receptors bind their ligand, they undergo a conformational change, and as a consequence, they bind histone acetylase complexes, thereby reversing their own repressing effects. In the presence of ligand, the ligand-bound conformation of the receptor also binds Mediator, stimulating preinitiation complex assembly.
In contrast to heterodimeric nuclear receptors, homodimeric receptors are found in the cytoplasm in the absence of their ligands. Hormone binding to these receptors leads to their translocation to the nucleus. The hormone-dependent translocation of the homodimeric glucocorticoid receptor (GR) was demonstrated in the transfection experiments shown in Figure 9-45a–c. The GR hormone-binding domain alone mediates this transport. Subsequent studies showed that in the absence of hormone, GR cannot be transported into the nucleus because its ligand-binding domain is partially unfolded by the major cellular chaperone Hsp70. As long as the receptor is confined to the cytoplasm, it cannot interact with target genes and hence cannot activate transcription. Hormone binding promotes a “handoff” of GR from Hsp70 to Hsp90, which, with coupled hydrolysis of ATP, refolds the GR ligand-binding domain, increasing the affinity for hormone and releasing GR from Hsp70 so that it can enter the nucleus. Once in the nucleus in the conformation induced by ligand binding, it can bind to response elements associated with target genes (Figure 9-45d). Once the receptor with bound hormone binds to a response element, it activates transcription by interacting with chromatin-remodeling and histone acetylase complexes and Mediator.
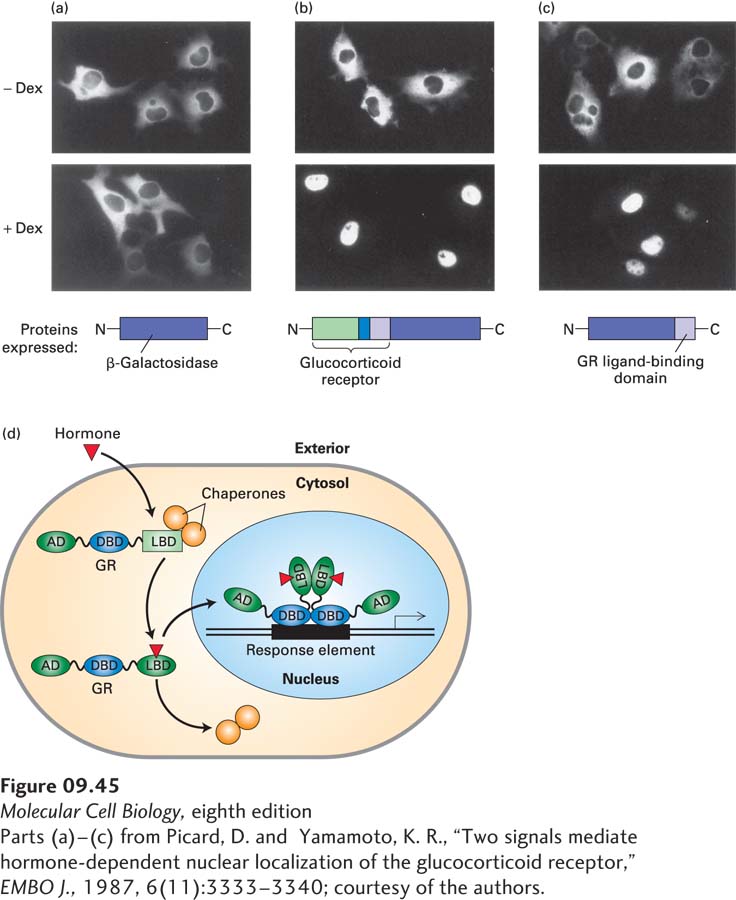
EXPERIMENTAL FIGURE 9-45 Fusion proteins demonstrate that the hormone-binding domain of the glucocorticoid receptor mediates translocation to the nucleus in the presence of hormone. Cultured animal cells were transfected with expression vectors encoding the proteins diagrammed at the bottom. Immunofluorescence with a labeled antibody specific for β-galactosidase was used to detect the expressed proteins in transfected cells. (a) In cells that expressed β-galactosidase alone, the enzyme was localized to the cytoplasm in the presence and absence of the glucocorticoid hormone dexamethasone (Dex). (b) In cells that expressed a fusion protein consisting of β-galactosidase and the entire glucocorticoid receptor (GR), the fusion protein was present in the cytoplasm in the absence of hormone but was transported to the nucleus in the presence of hormone. (c) Cells that expressed a fusion protein composed of β-galactosidase and only the GR ligand-binding domain (light purple) also exhibited hormone-dependent transport of the fusion protein to the nucleus. (d) Model of hormone-dependent gene activation by a homodimeric nuclear receptor. In the absence of hormone, the receptor is kept in the cytoplasm by interaction between its ligand-binding domain (LBD) and chaperone proteins. When hormone is present, it diffuses through the plasma membrane and binds to the ligand-binding domain, causing a conformational change that releases the receptor from the chaperone proteins. The receptor with bound ligand is then translocated into the nucleus, where its DNA-binding domain (DBD) binds to response elements, allowing the ligand-binding domain and an additional activation domain (AD) at the N-terminus to stimulate transcription of target genes.
[Parts (a)–(c) from Picard, D. and Yamamoto, K. R., “Two signals mediate hormone-dependent nuclear localization of the glucocorticoid receptor,” EMBO J., 1987, 6(11):3333–3340; courtesy of the authors.]
