Many Bacterial Responses Are Controlled by Two-Component Regulatory Systems
As we have just seen, control of the E. coli glnA gene depends on two proteins, NtrC and NtrB. Such two-component regulatory systems control many responses of bacteria to changes in their environment. At high concentrations of glutamine, glutamine binds to a sensor domain of NtrB, causing a conformational change in the protein that inhibits its histidine kinase activity (Figure 9-6a). At the same time, the regulatory domain of NtrC blocks its DNA-binding domain from binding the glnA enhancers. At low concentrations of glutamine, glutamine dissociates from the sensor domain in the NtrB protein, leading to activation of a histidine kinase transmitter domain in NtrB that transfers the γ-phosphate of ATP to a histidine residue (H) in the transmitter domain. This phosphohistidine then transfers the phosphate to an aspartic acid residue (D) in the NtrC protein. This causes a conformational change in NtrC that unmasks the NtrC DNA-binding domain so that it can bind to the glnA enhancers.
Many other bacterial responses are regulated by two proteins with homology to NtrB and NtrC (Figure 9-6b). In each of these regulatory systems, one protein, called a histidine kinase sensor, contains a latent histidine kinase transmitter domain that is regulated in response to environmental changes detected by a sensor domain. When activated, the transmitter domain transfers the γ-phosphate of ATP to a histidine residue in the transmitter domain. The second protein, called a response regulator, contains a receiver domain homologous to the region of NtrC containing the aspartic acid residue that is phosphorylated by activated NtrB. The response regulator contains a second functional domain that is regulated by phosphorylation of the receiver domain. In many cases, this domain of the response regulator is a sequence-specific DNA-binding domain that binds to related DNA sequences and functions either as a repressor, like the lac repressor, or as an activator, like CAP or NtrC, regulating the transcription of specific genes. However, the effector domain can have other functions as well, such as controlling the direction in which the bacterium swims in response to a concentration gradient of nutrients. Although all transmitter domains are homologous (as are receiver domains), the transmitter domain of a specific sensor protein will phosphorylate only the receiver domains of specific response regulators, allowing specific responses to different environmental changes. Similar two-component histidyl-aspartyl phospho-relay regulatory systems are also found in plants.
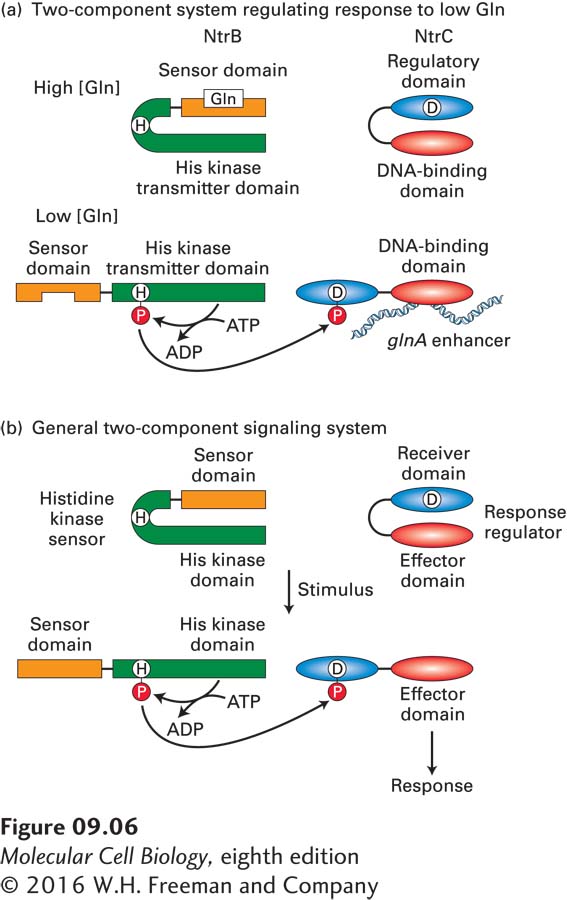
FIGURE 9-6 Two-component regulatory systems. (a) At low cytoplasmic concentrations of glutamine, glutamine dissociates from NtrB, resulting in a conformational change that activates a protein kinase transmitter domain that transfers an ATP γ-phosphate to a conserved histidine (H) in the transmitter domain. This phosphate is then transferred to an aspartic acid (D) in the regulatory domain of the response regulator NtrC. This converts NtrC into its activated form, which binds the enhancer sites upstream of the glnA promoter (see Figure 9-5). (b) General organization of two-component histidyl-aspartyl phospho-relay regulatory systems in bacteria and plants. See A. H. West and A. M. Stock, 2001, Trends Biochem. Sci. 26:369.
