Chapter 10. Transcriptional Regulation in Herpes Simplex Virus
Introduction

Analyze the Data 10-1: Transcriptional Regulation in Herpes Simplex Virus
Most humans are infected with herpes simplex type I virus (HSV-I), the causative agent of cold sores. The HSV-I genome comprises about 100 genes, most of which are expressed in infected host cells at the site of oral sores. The infection process involves replication of viral DNA, transcription and translation of viral genes, assembly of new viral particles, and death of the host cell as the viral progeny are released. Unlike most other types of viruses, herpesvirus also has a latent phase, in which the virus remains hidden in neurons. These latently infected neurons are the source of active infections, causing cold sores when latency is overcome.
Interestingly, only a single viral transcript is expressed during latency. This transcript, LAT (latency-associated transcript), does not encode a protein, and neurons infected with mutant HSV-I lacking the LAT gene undergo cell death by apoptosis at a rate twice that of cells infected with wildtype HSV-I. To determine if LAT functions to block apoptosis by encoding a miRNA, the following studies were done (see Gupta et al., 2006, Nature 442:82–85).
Question
a. A human cell line was transfected with an expression vector that expresses the Pst-Mlu fragment of the LAT gene (see diagram in part b). The percentage of these transfected cells that then underwent apoptosis when exposed to an apoptosis-inducing drug was compared with that of control cells. The experiment was repeated in cells in which Dicer expression was knocked down using Dicer siRNA. The data obtained are shown in the graph below. What conclusions can be drawn from these data? Why did the scientists who conducted this study examine the effects of silencing Dicer?
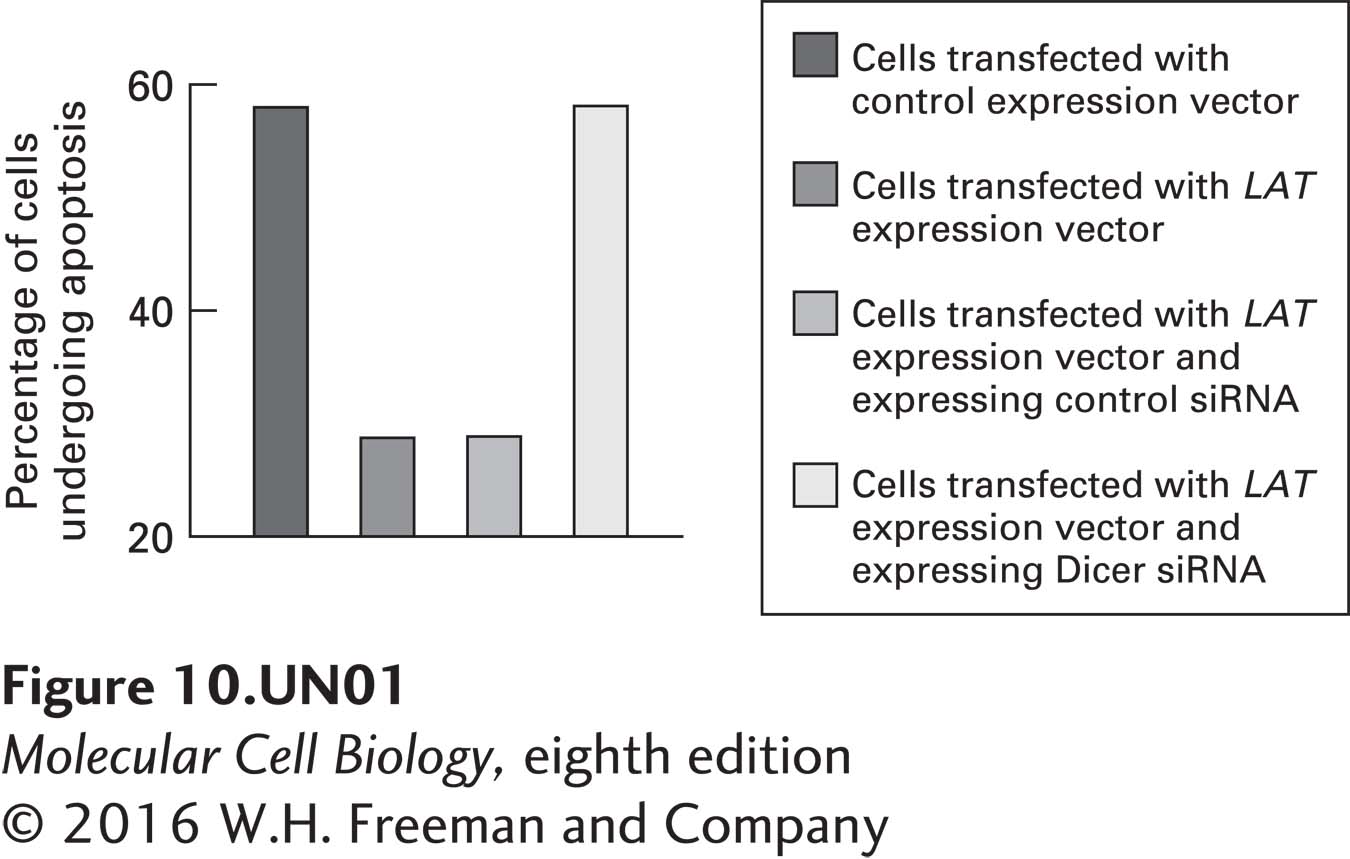
Question
b. Cells were transfected with an expression vector expressing the Pst-Mlu fragment of the LAT gene from which the region between the two Sty restriction siteshad been deleted (ΔSty; see diagram below). When these cells were induced to undergo apoptosis, they died at the same rate as did nontransfected cells. In additional studies, cells were transfected with an expression vector expressing the Sty-Sty region of the LAT gene. These cells exhibited the same resistance to apoptosis as did cells transfected with the Pst-Mlu fragment. What can be deduced from these findings about the region of the LAT gene required to protect cells from apoptosis?
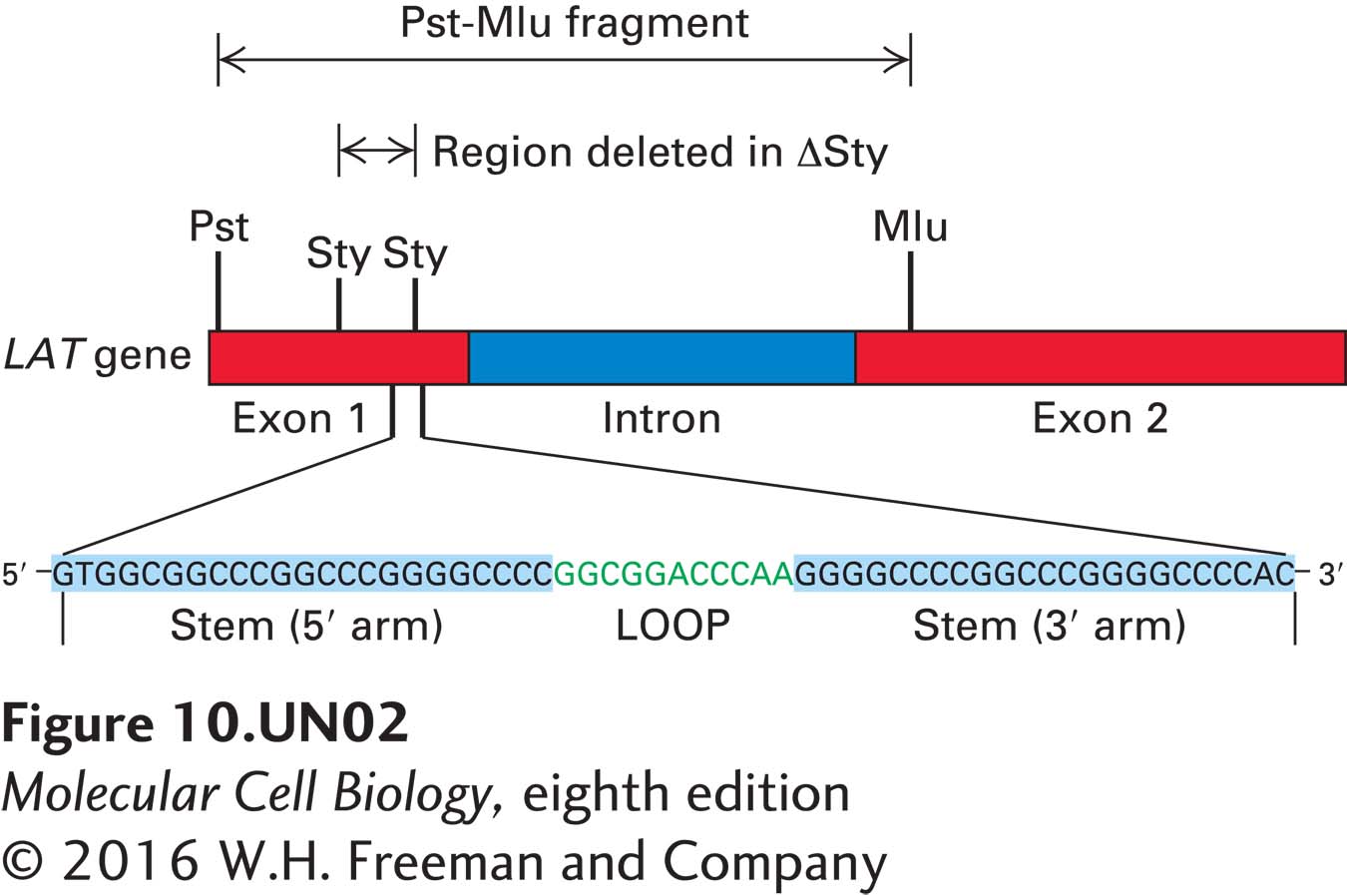
Question
c. RNA encoded within the Sty-Sty region is predicted to form a stem loop (see diagram in part b). Northern blot analysis was performed on total cellular RNA isolated from control cells, cells infected with wild-type HSV-I, cells infected with an HSV-I deletion mutant from which the sequence between the two Sty sites in the LAT gene was deleted (ΔSty), and cells infected with a rescued ΔSty virus into which the deleted region had been reinserted (StyR). The probe used for the Northern blot was the labeled 3′ stem region of the LAT RNA in the Sty-Sty region, as diagrammed in part (b). The RNAs recognized by this probe were either 55 nucleotides or 20 nucleotides long, as shown in the Northern blot below. Why were two different-sized RNAs detected? When a second probe was used that was the labeled 5′ stem region of the RNA sequence shown in part (b), only the 55-nucleotide RNA was detected. What can you deduce about the processing of RNA expressed from the LAT gene? What enzyme probably produced the 55-nucleotide RNA? In what part of the cell? What enzyme probably produced the 20-nucleotide RNA? In what part of the cell?
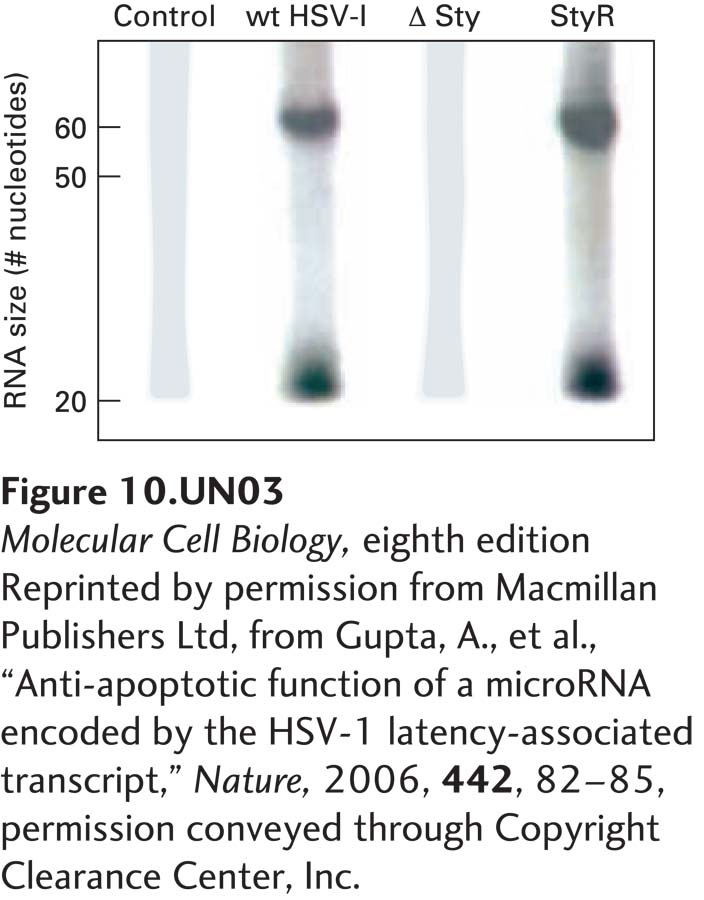
Question
d. TGF-β mRNA encodes a protein, transforming growth factor β, that inhibits cell growth and induces apoptosis. The 3′ untranslated region (3′ UTR) of TGF-β mRNA can form an imperfect duplex with miRNA encoded by the 5′ stem region of the LAT Sty-Sty domain (miR-LAT), as shown below. In what way might the expression levels of TGF-β differ in cells infected with wild-type HSV-I from those in uninfected cells? What can you infer about latent HSV-I infections from these studies?
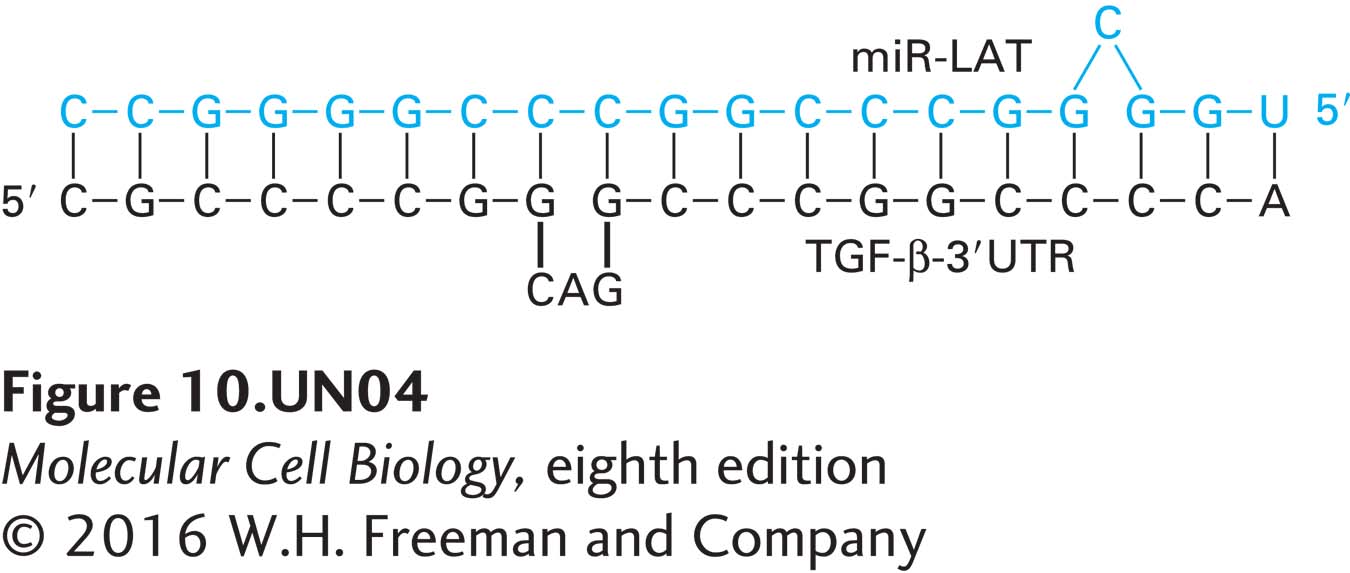
Activity results are being submitted...