Chapter 14. Specificity of Membrane Fusion Encoded in SNARE Proteins
Introduction

Analyze the Data 14-1: Specificity of Membrane Fusion Encoded in SNARE Proteins
In order to examine the specificity of membrane fusion conferred by specific v-SNAREs and t-SNAREs, researchers reconstituted liposomes (artificial lipid membranes) with specific t-SNARE complexes or with v-SNAREs (see McNew et al., 2000, Nature 407:153–159). To measure fusion, the v-SNARE liposomes also contained a fluorescent lipid at a relatively high concentration such that its fluorescence is quenched. (Quenching is reduced fluorescence relative to that expected. In this case, quenching occurs because the fluorescent lipids are too concentrated and interfere with one another’s ability to become excited.) On fusion of these liposomes with those lacking the fluorescent lipids, the fluorescent lipids are diluted, and quenching is alleviated. Three sets of liposomes were prepared using yeast t-SNARE complexes: those containing plasma membrane t-SNAREs, Golgi t-SNAREs, or vacuolar t-SNAREs. Each of these was mixed with fluorescent liposomes containing one of three different yeast v-SNAREs. The following data were obtained.
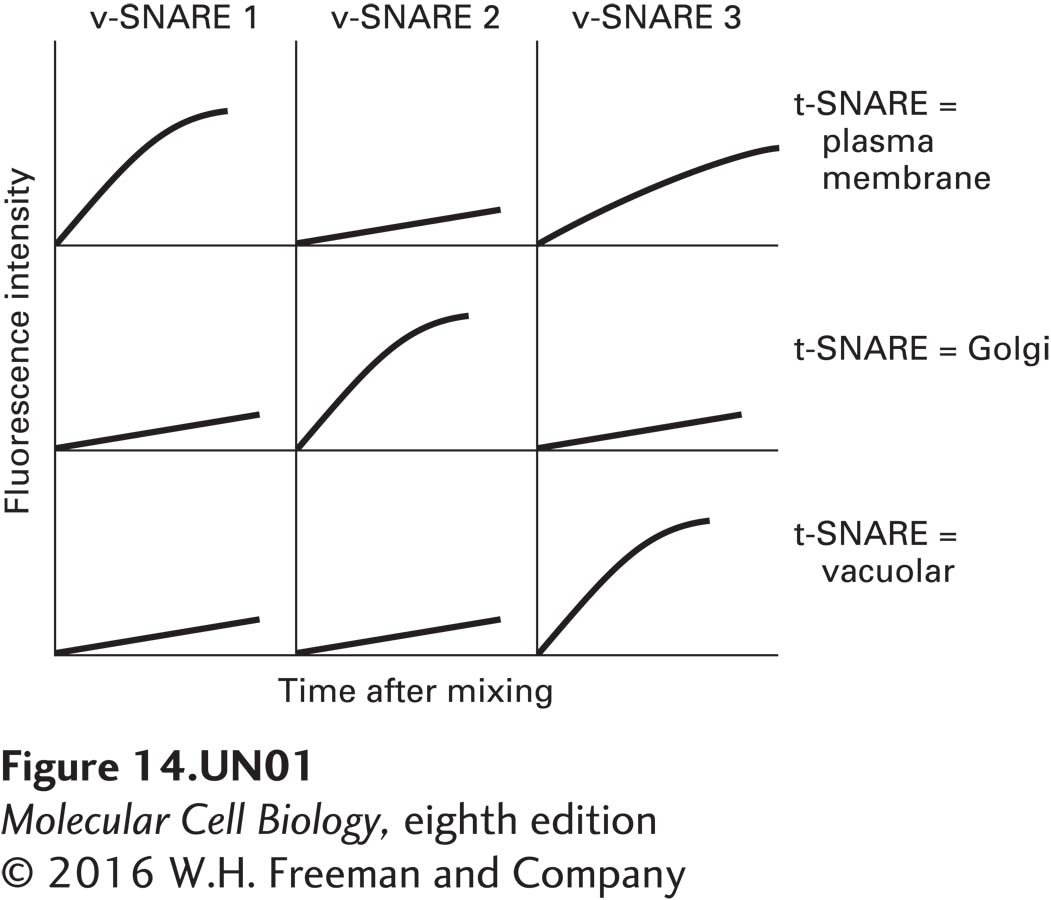
Question
7YinBr+EX1hFxrvBydX0y1WgT8yeXWtKHWsXAIpVMrgMkPqVJcfm7ab14P/+plbcQcSM2ruOyUDZrsDVPnEc5gkjYMedImutUAb8Qg9fv4qe7So51hN8JksSTmsgfPifPOaR7PQMZejqLHBgaRP+kQxTIraGKYFTNDGjIrFDG30=Question
CwVScqt41NyRZ3L4A5YAJMt4zyv+2tWwgyRCq0ikp5S1zs94xyH4PzZORSaC3Z9pLXd0/ECmDVik8x+1GVBrPml/+473Pm65dtdvgn0HV/JdEbevjF3u+Z6kYcJ1YpOSZfMhM37gyTc=Question
uZnIiWXMoGgUgINMDFU9GUeJG5xpNNV5zAQpfdMxny0TBAKaltVw2U8v3nb376jEEt6XZK0d3152+37UiWQh9P1THIT+fHOuLKtih0fyE2N41o3uMjr/Lxz4SP5as6y0NdE8EoC3Uj3tXEd0Eg9fr4p67HIGxQ3g3k307qDIaGxgcpZw9D4L44r5VieMFYk/dEJVzrvRK7cDx0Fb9qDQ8oMyS14=Question
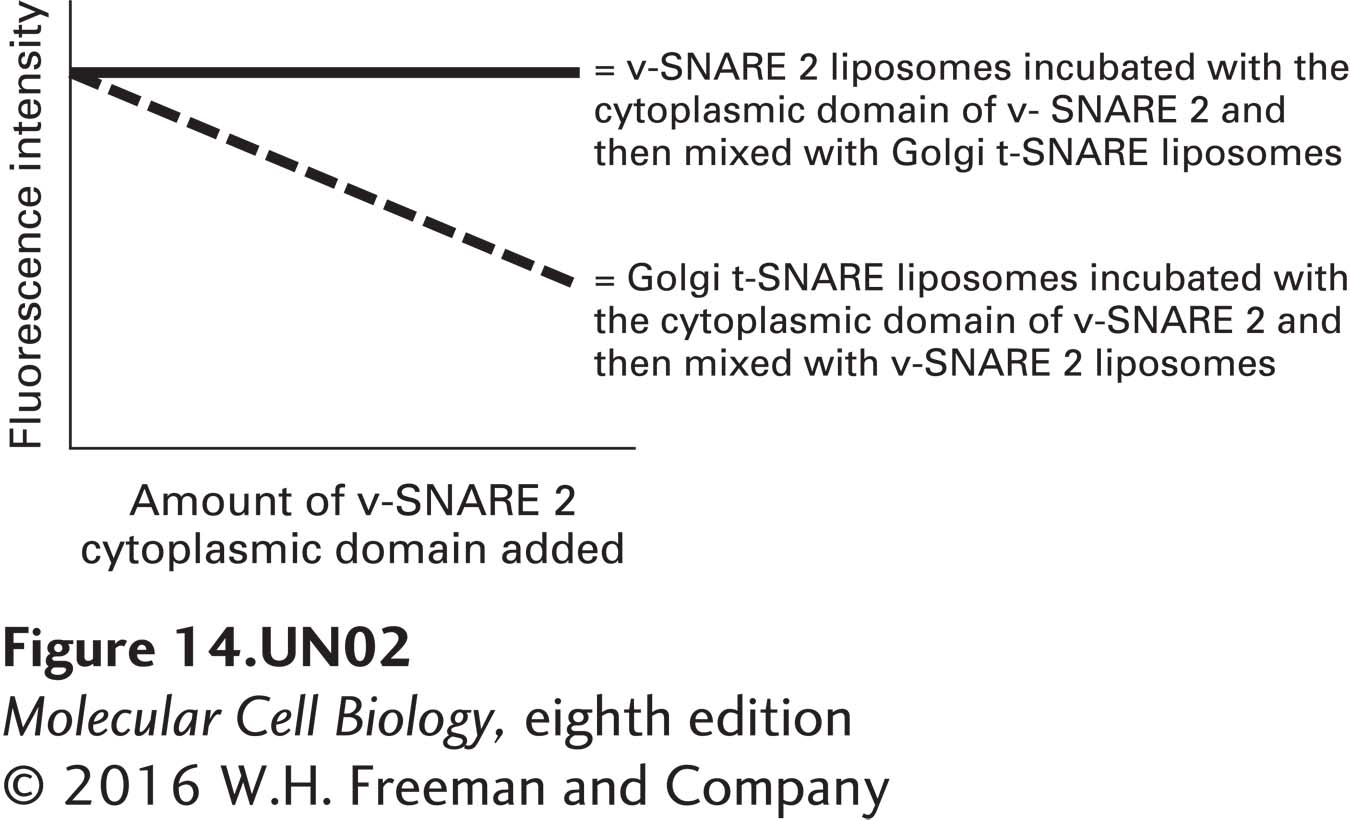
d. The cytoplasmic domain of v-SNARE 2 has been expressed and purified from E. coli. Various amounts of this domain were incubated either with Golgi t-SNARE liposomes or with v-SNARE 2 liposomes. The liposomes were then washed free of unbound protein. The two types of liposomes were then mixed, as indicated below, and the fluorescence of each sample was measured 1 hour after mixing. How can the data in the graph below be explained? What would you predict the outcome to be if yeast were to overexpress the cytoplasmic domain of v-SNARE 2?
Activity results are being submitted...