Chapter 15. G Protein Mutations in Acromegaly
Introduction

Analyze the Data 15-1: G Protein Mutations in Acromegaly
Mutations in heterotrimeric G proteins can cause many diseases in humans. Patients with acromegaly often have pituitary tumors that oversecrete growth hormone (GH). Growth hormone–releasing hormone (GHRH) stimulates GH release from the pituitary by binding to GHRH receptors there and activating adenylyl cyclase. Researchers wanted to know whether mutations in the Gαs coupled to the GHRH receptor played a role in this condition. Cloning and sequencing of the wild-type Gαs gene from normal individuals and a mutant Gαs gene from patients with the pituitary tumors revealed a missense mutation in the Gαs gene sequence.
Question
a. To investigate the effect of the mutation on Gαs activity, wild-type and mutant Gαs cDNAs were transfected into cells that lack the Gαs gene. These cells express a β2-adrenergic receptor that can be activated by isoproterenol, a β2-adrenergic receptor agonist. Membranes were isolated from transfected cells and assayed for adenylyl cyclase activity in the presence of GTP or the hydrolysis-resistant GTP analog GTP-γS. From the figure below, what do you conclude about the effect of the mutation on Gαs activity in the presence of GTP alone compared with GTP-γS alone or GTP plus isoproterenol (iso)?
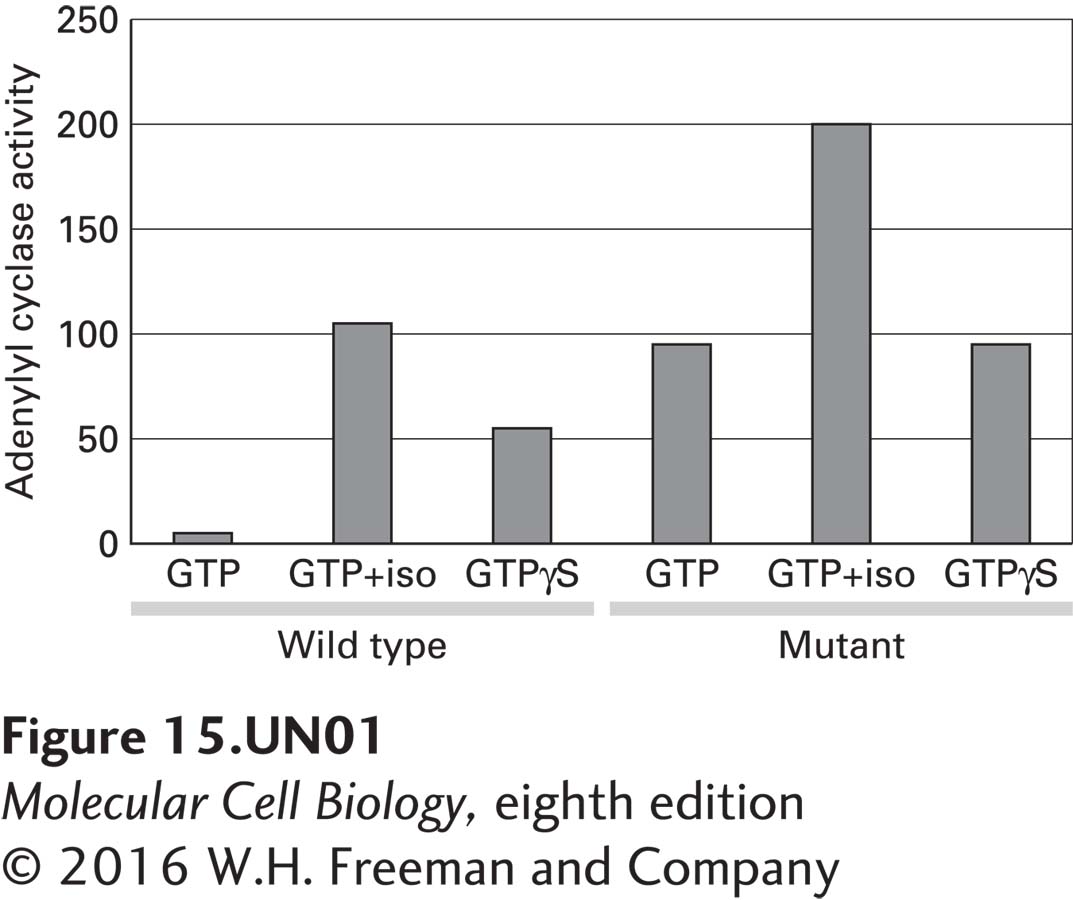
For the wild-type G protein, the activity of adenylyl cyclase is what you would expect. In the presence of GTP, there is a basal level of adenylyl cyclase activity, which can be greatly stimulated by the addition of isoproterenol. Isoproterenol binds to the β2-adrenergic receptor and causes activation of adenylyl cyclase. In comparing adenylyl cyclase activity in the presence of GTP or GTP-γS, again the expected result is seen. The addition of GTP-γS leads to an increase in adenylyl cyclase activity because GTP-γS is nonhydrolyzable. Thus, the Gαs subunit remains active, leading to prolonged activation of adenylyl cyclase. In the case of the mutant, again the addition of isoproterenol results in an increase in adenylyl cyclase activity as expected. The adenylyl cyclase activity, however, is not different in the presence of GTP or GTP-γS. Thus, the mutation causes an increase in the basal activity of adenylyl cyclase, likely due to a change in the GTPase activity.
Question
WYIGQWoEJZ7MyQwjyrezhPUPUYN4Vyh4L9AqwG5zgeIk7suSwf7HL0yzTqGUto5e8ykH2eNjQ09qyDggAZzkahN9V7tInBveQBFxtYkl5sRjNbJYc805i4ilLHvt7xtGZe/BPlXvRfhjCxcLCCLPx7a9En8P0VNdwyh8Ji6nVKyqJV0evGMc0RNpjRNWQTVtpCWfOL3AKZ/sK8ararLdFT2A4b2Q4ggt/s/cs/fyUAyywR2/sqUjRXa7gPnXOJ5sXn65qO/VrmMDQZ++YYun8PbEyhJAuwkLWeEelzON31NK/u2eMbRYvBkE5aD1Jakgu0vo9C5yOvou6FpjQ1FcjoB182le7Mvh8SmO9BtFBw+w4ROKkPlR5A==In cells transfected with the mutant G protein, higher levels of adenylyl cyclase would be present relative to cells transfected with wild-type G protein. Thus, mutant-transfected cells would have higher cAMP levels, which would result in higher levels of active protein kinase A. The higher protein kinase A levels would result in more extensive phosphorylation of target proteins, which would affect normal cell development and proliferation.
Question
c. To further characterize the molecular defect caused by this mutation, the intrinsic GTPase activity present in both wild-type and mutant Gαs was assayed. Assays for GTPase activity showed that the mutation reduced the kcat-GTP (catalysis rate constant for GTP hydrolysis) from a wild-type value of 4.1 min−1 to the mutant value of 0.1 min−1. What do you conclude about the effect of the mutation on the GTPase activity present in the mutant Gαs subunit? How do these GTPase results explain the adenylyl cyclase results shown in part (a)?
LVpEj/AU+QIqOftjhA3/fXeCtqcJejMc3Pnb1CUEVeZfMtdS8cu+LQ5UwzJRnGomgcrfIFvA3gCtwd11/t0kmI1Wg/VjoEzz4ttwxAfrDBMb1qE84e45F36b+nNBhXj71wLCeqYzfzi2XTzbeplovgAoxq8mw5+Yu70qgK9uKYx7ZMmifOW4hZoKZLrQJYpUP7oQ4WxBfp1RTUPIeypgzNsdR5TJXj+SEx3RFh0XLb0s88Y+p9aL1xAiqQaE2vie5uzwyMOflY/Mjfm4y2sb6gHKmT5uhjO3rjSBGT2IoL9zP4648u8ziw==From the GTPase results, it is clear that the mutation affects the intrinsic GTPase activity of the Gas subunit. These results are consistent with the adenylyl cyclase results. For the mutant G protein, binding GTP or GTP-γS to the Gαs subunit leads to the same level of adenylyl cyclase activation because the Gαs subunit has greatly reduced ability to cleave GTP.
Activity results are being submitted...