Appendix A: Basic Chemistry
Appendix A
Basic Chemistry
A-
matter Anything that occupies space and has mass; exists in three main physical states: as a solid, liquid, or gas.
Understanding the material basis of ecosystems and economic systems is improved by delving beneath the surface layers of matter to consider some details of chemistry, the science concerned with the composition, structure, properties, and reactions of matter. A logical entry point into a discussion of basic chemistry is the atom.
Atoms
atom The smallest particle of a pure substance that still retains the properties of the substance.
nucleus The massive central core of an atom, which is made up of protons and neutrons, around which the atom’s electrons move.
proton A subatomic particle, found in the nucleus of atoms, that has a positive charge (+1) and atomic mass of 1.
electron A subatomic particle with a negative charge (–1) and a mass approximately 1/2,000 that of a proton or neutron.
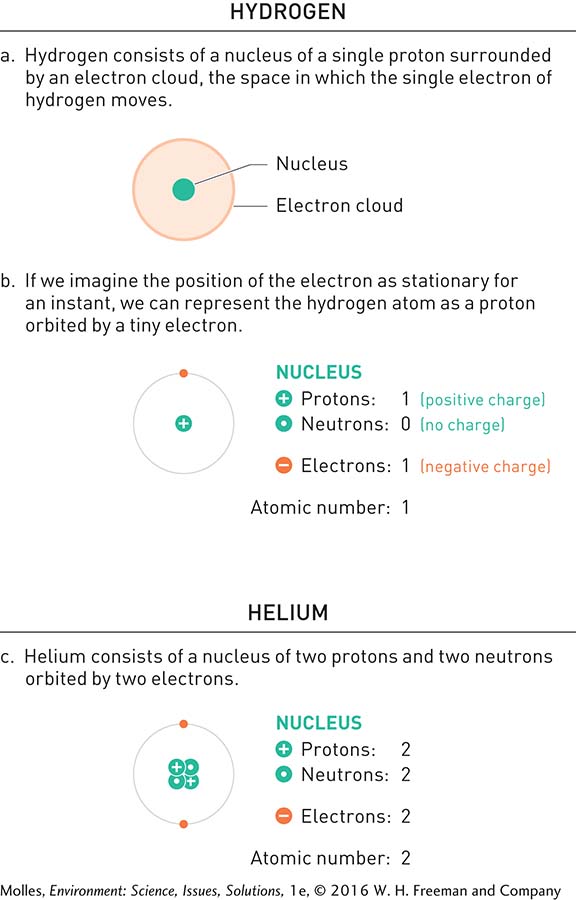
The basic constituent of all matter is the atom, which is the smallest particle of a pure substance that still retains the chemical and physical properties of that substance. The simplest and most abundant atom in the universe is hydrogen. Hydrogen’s nucleus, the central massive region of an atom, contains a single proton orbited by a single electron (Figure A.1). Because they have equal and opposite electrical charges, protons (+1) and electrons (–1) are attracted to each other and cancel each other out electrically. As a consequence, the hydrogen atom with its single proton and single electron is electrically neutral [(+1) + (–1) = 0].
neutron A subatomic particle, found in the nucleus of atoms, that has a mass approximately equal to that of a proton but no electrical charge.
atomic weight The average mass of the atoms in a naturally occurring pure substance (e.g., gold, carbon, oxygen).
Because an electron moves at nearly the speed of light, it forms an electron cloud around the nucleus, which is one way to represent the orbit of an electron around the nucleus (Figure A.1a). For convenience, however, we can mentally freeze the position of an electron and picture it as moving in a fixed orbit (Figure A.1b). Notice that the helium atom (Figure A.1c) has two protons (+2 charge) and two electrons (–2 charge), which again exactly balance each other electrically. However, the helium nucleus also includes two neutrons, which are equal in weight to protons but have no electrical charge. Therefore, neutrons contribute to atomic weight but not to the electrical properties of atoms. Meanwhile, because it takes approximately 2,000 electrons to equal the mass of a single neutron or proton, electrons contribute to the electrical properties of atoms but insignificantly to their mass.
element A substance composed of a single type of atom (e.g., hydrogen, helium, iron, or lead).
atomic number The number of protons in the nucleus of an atom of an element; equal to the number of electrons in a neutral atom.
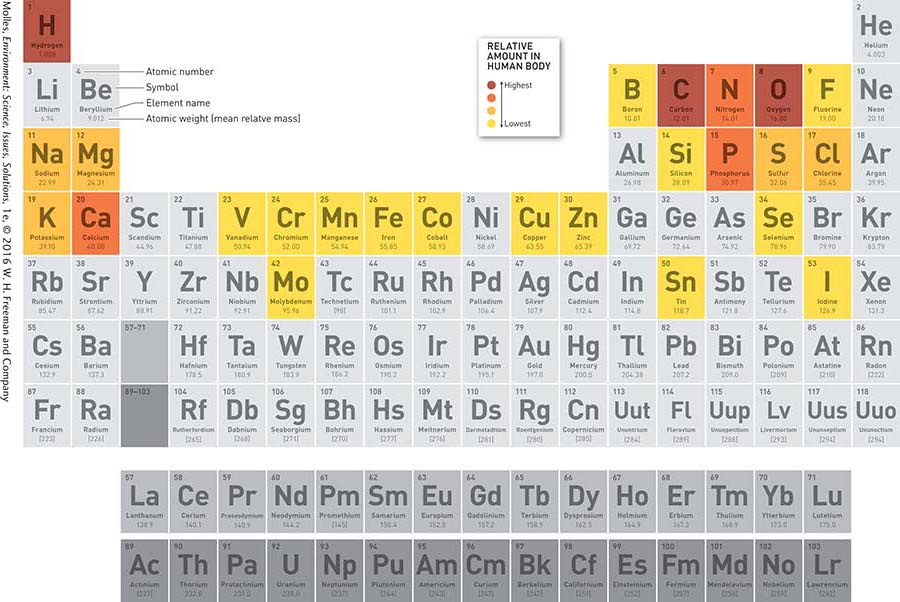
Substances composed of a single type of atom, such as hydrogen or helium, are called elements. Elements that we commonly encounter in everyday life include the oxygen in the air we breathe, the aluminum in a soft drink can, and the copper in a penny. The elements are traditionally arranged into a table called the periodic table, which orders elements by number of protons, or atomic number, and groups elements into families of substances with similar chemical properties (Figure A.2).
A-
The atomic structure of elements provides clues regarding their chemical properties. Table A.1 shows the atomic structures of 11 biologically important elements. Just six of these—
A-
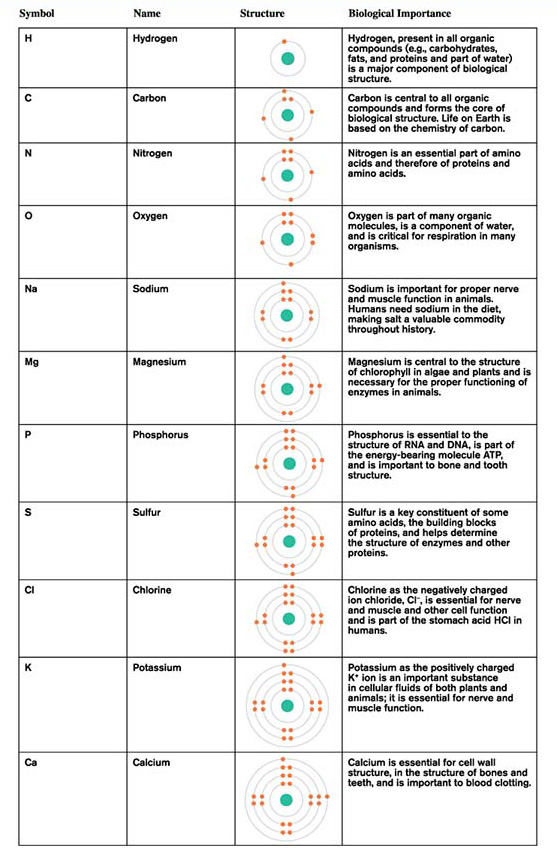
The basic chemistry of the elements is strongly influenced by the number of electrons in the outermost electron shell. Those elements with few electrons in the outer electron shell tend to give up, or donate, electrons to other elements, while elements with nearly a full complement of electrons in their outer electron shell tend to accept electrons from other elements. For instance, hydrogen, sodium, and potassium tend to give up the single electron in their outermost shell, and magnesium and calcium give up the two electrons in their outer electron shells. Meanwhile, oxygen and sulfur accept two electrons to fill their outermost electron shells, and chlorine accepts one. What about an element such as carbon, with an outer shell occupied by four electrons, exactly half the full complement of eight? Such elements tend to share electrons with other elements.
Molecular Structure and Chemical Reactions
A-
molecule A particle consisting of two or more atoms held together by chemical bonds; the constituent atoms may be of the same or different elements.
compound A substance composed of a fixed ratio of two or more elements (e.g., water consists of two hydrogen atoms and one oxygen atom: H2O); compounds can be broken down into the elements of which they are made through chemical or physical processes.
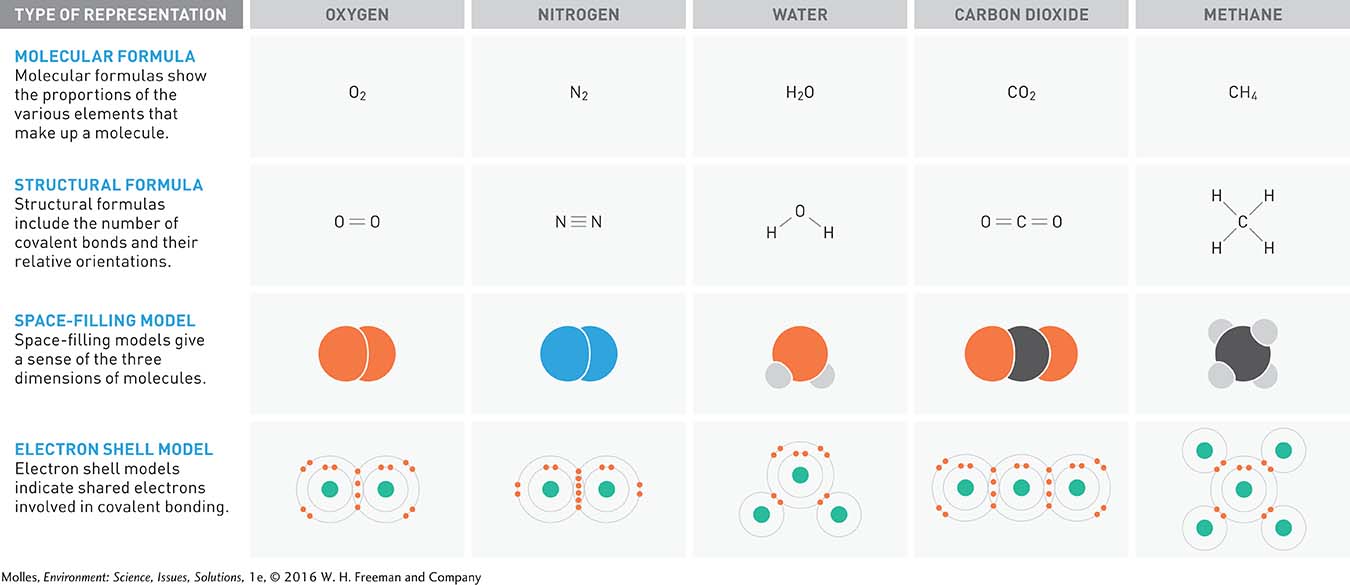
As atoms interact, donating, accepting, or sharing electrons, they form substances called molecules. Molecules made up of atoms of two or more different elements are compounds. Figure A.3 shows four common ways to represent molecules. The representation you use to illustrate a molecule will depend on the information you want to convey and on convenience. The molecular formula, showing the proportions of each of the elements making up a molecule, is easy to write. The structural formula includes the number of bonds and their relative positions. A space-
chemical reaction A process by which new substances are produced via the rearrangement of the atoms undergoing the reaction, generally through the exchange or sharing of electrons.
covalent bond A bond between atoms that share one or more pairs of electrons.
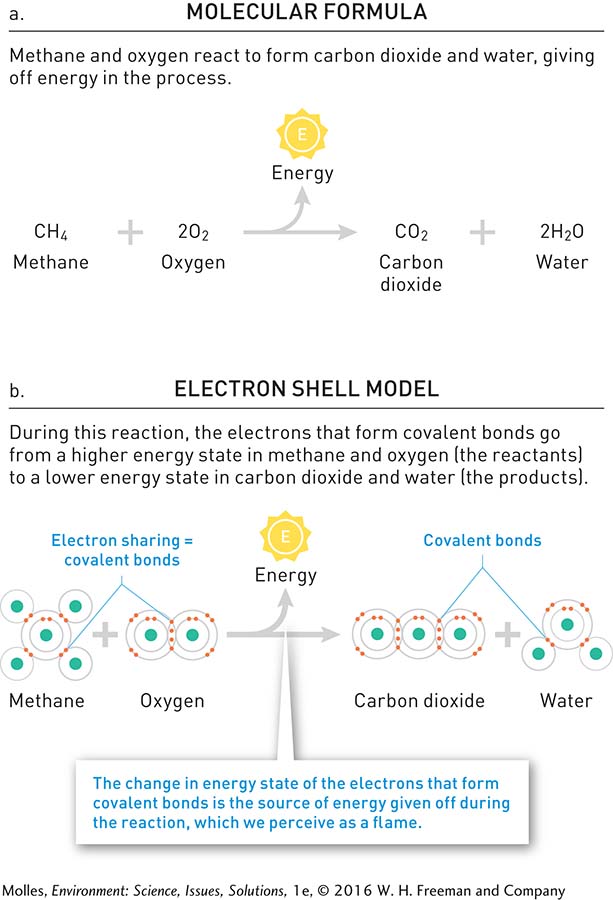
The discussion so far has included a wide variety of chemical compounds that occur in our own bodies and in the world around us. How do such chemical compounds form? Compounds form as the products of chemical reactions between elements, between compounds, or between compounds and elements. Figure A.4 outlines a chemical reaction in which methane gas, the main ingredient of natural gas, reacts with oxygen to produce carbon dioxide and water. During the burning of natural gas, the electrons forming the covalent bonds in both methane molecules and oxygen molecules are broken and reformed. The bond electrons are rearranged to form covalent bonds between carbon and oxygen and between oxygen and hydrogen, yielding carbon dioxide and water as products of the reaction. This reaction releases considerable heat and light as the electrons involved in bond formation go from higher energy states in the reactants (methane and oxygen) to lower energy states of the products (water and carbon dioxide). The heat from this reaction is commonly used to warm homes and cook food.
The compounds produced by some reactions do not involve covalent bonds. Figure A.5 shows the reaction between sodium (a highly reactive, soft metal) and chlorine (an irritating, greenish gas). The product of this reaction, sodium chloride (better known as table salt), has properties that are quite different from the elements that combined to form it. The upper panel of Figure A.5 shows the reaction using the molecular formulas for sodium and chlorine, while the lower panel uses electron-
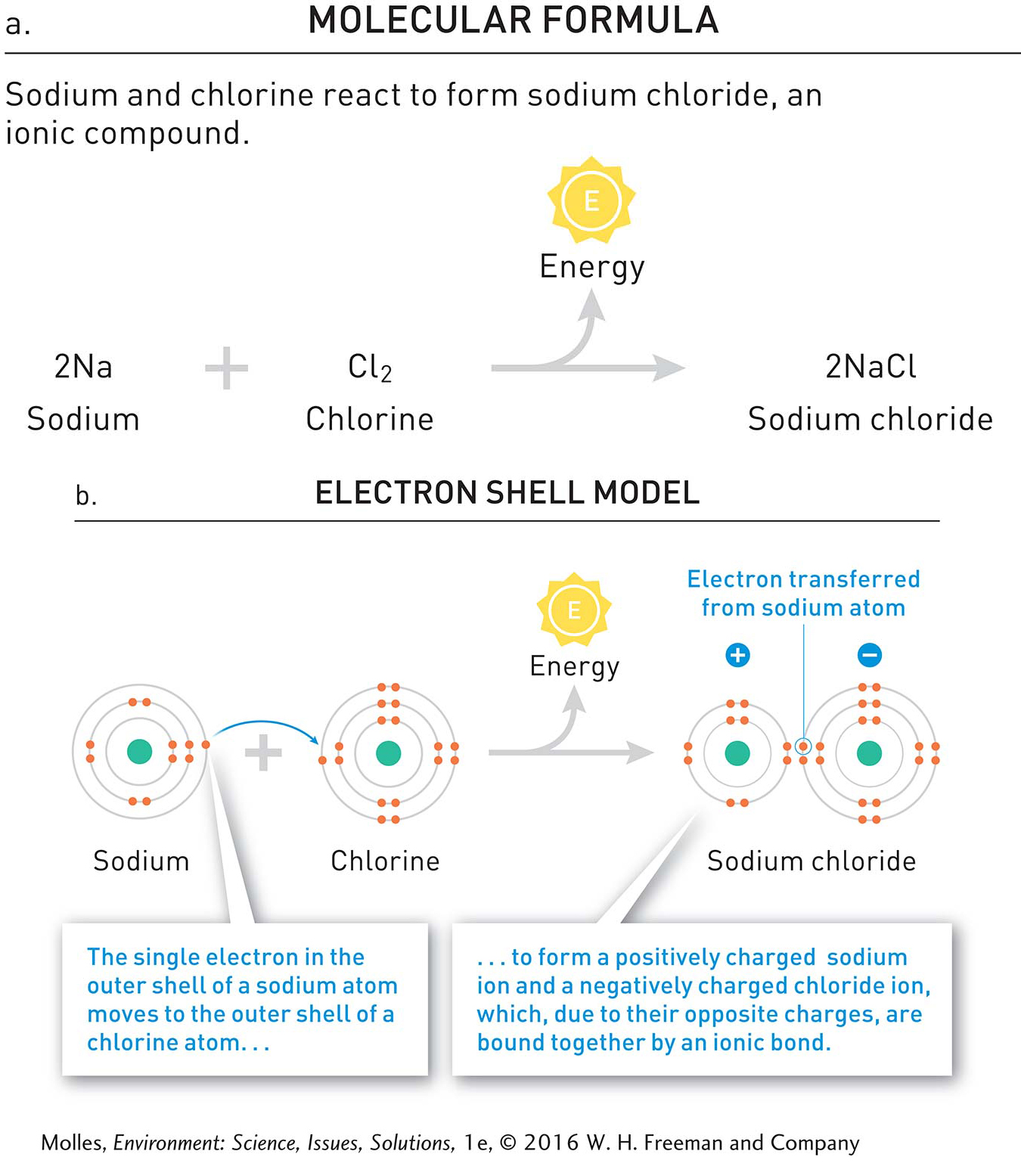
ion An atom, or group of atoms, with a net positive or negative charge (e.g., chloride, Cl–, and sodium, Na+, ions).
A-
During the formation of sodium chloride, the single electron in the outermost electron shell of sodium moves from the sodium atom to the chlorine atom, releasing energy. In the process, the sodium atom is converted into a sodium ion with a charge of +1. During this reaction, the chlorine atom is converted to a chloride ion with a charge of –1.
ionic bond A chemical bond involving the attraction between two oppositely charged ions.
Where do these charges come from? Sodium atoms have an atomic number of 11, which means that they have exactly 11 protons in their nucleus. When one of the sodium electrons moves to chlorine, the remaining 10 electrons do not quite cancel out the total positive charge of the 11 nuclear protons. Consequently, the sodium ion has a net charge of +1. Similarly, with the addition of an electron, 18 electrons orbit the chloride ion with its 17 protons, producing a net charge of –1. Because they have opposite charges, sodium and chloride ions are attracted to each other. The result of this attraction is an ionic bond between sodium and chloride ions.
Cells and Vital Molecules
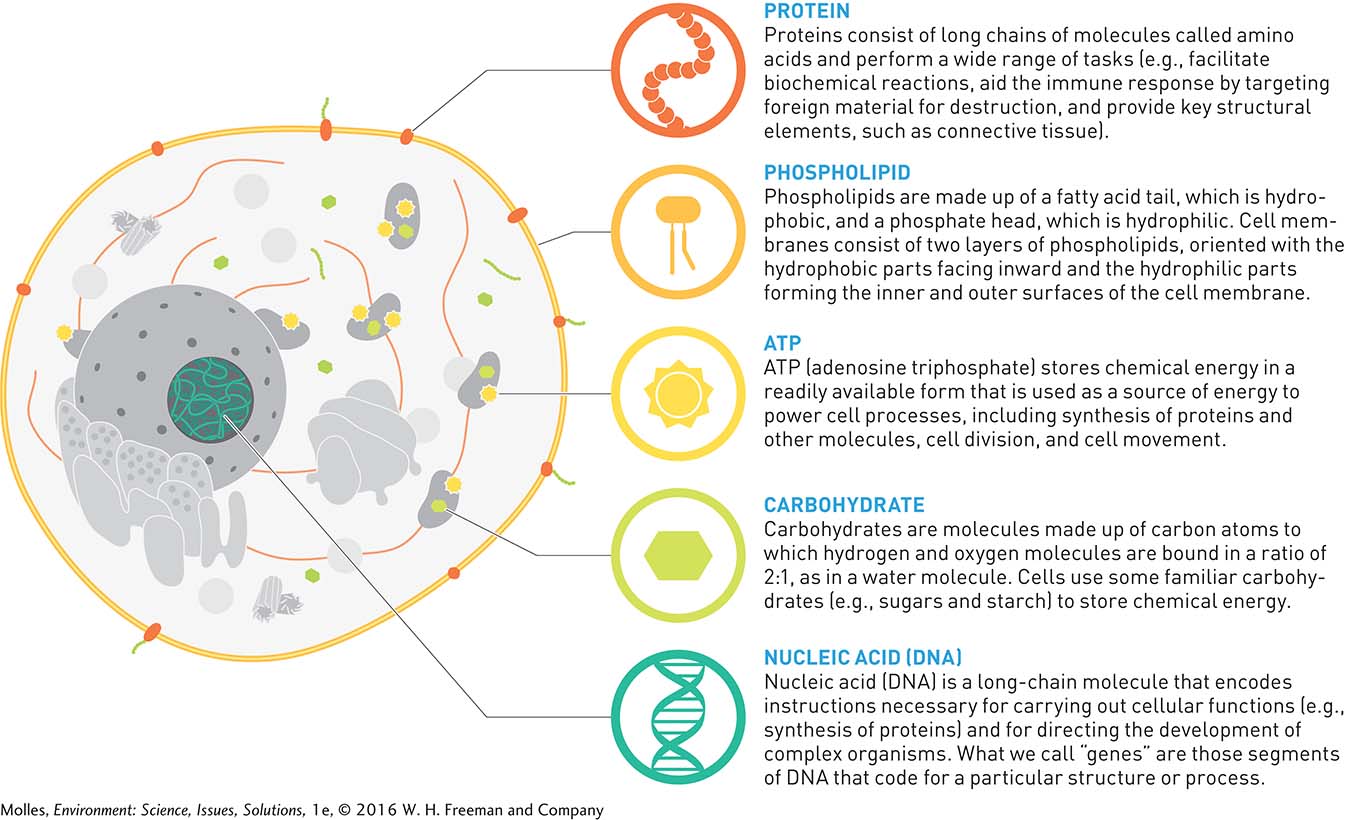
All the life forms on Earth, from bacteria and algae to whales and redwood trees, are built of individual units called cells (Figure A.6). The structure and functioning of cells is the product of the molecules of which they are made. Though atoms can combine in innumerable ways to form endless varieties of molecules, only a few types of molecules are required to form the great diversity of life on Earth. Those key molecules are the basis for the structure, energetics, and reproduction of all organisms.
lipids Organic molecules composed of long chains of carbon atoms bonded mainly to hydrogen (e.g., fats, oils, or waxes); important components of cell membranes; function as energy storage molecules in animals and plants.
proteins Long chains of amino acids (i.e., molecules consisting mainly of carbon, nitrogen, hydrogen, and oxygen) that control rates of chemical reactions, provide structural support, and perform many other functions.
DNA (deoxyribonucleic acid) The carrier of genetic information in cells, consisting of two complementary chains of molecules, called nucleotides, wound in a double helix; this source of biological inheritance, passed from parents to offspring, directs the development and functioning of an organism.
ATP (adenosine triphosphate) A molecule made up of adenine bonded to three phosphate molecules that releases energy when one of the bonds between phosphate molecules is broken; ATP is the main energy source for cells.
Examples of the main molecules making up living systems are arrayed around the animal cell illustrated in Figure A.6. Cells are separated from the surrounding environment by a membrane that is selectively permeable. Within a cell are a variety of organelles, also bound by a membrane, that perform essential functions in the life of the cell. The cell membrane is constructed of lipids with embedded proteins, consisting of chains of amino acids. The cell nucleus contains DNA (deoxyribonucleic acid), the repository of genetic information in the cell. That information is carried to the ribosomes, where proteins are assembled. The mitochondria are sites where the energy in sugars and fats are converted to ATP (adenosine triphosphate), a common energy source for cell processes.
Summary
A-
Everything in the universe is composed of matter, which exists as a solid, liquid, or gas. The basic constituent of all matter, the atom, is the smallest particle of a substance that retains its properties. Atoms react chemically—