11.6 Infectious diseases spill over from wild species and continue evolving to evade our defenses
344
The infectious diseases that plague human societies emerged from natural ecosystems. Disease epidemics can often be linked to environmental problems and, sometimes, our misguided attempts to solve them.
Ebola, Deforestation, and Bushmeat
On December 26, 2013, an 18-
zoonotic disease Any infectious disease that can spread from animals to humans.
bushmeat The butchered meat of wild animals, most commonly from African forests.
Ebola is a zoonotic disease, which is any infectious disease that can spread from animals to humans. According to the U.S. CDC, 6 out of 10 infectious diseases are zoonotic diseases. When humans come in close contact with wild animals through the pet trade or when they kill and butcher wild animals for so-
Deforestation in tropical countries also puts humans in closer contact with wild animals. Scientists have found Ebola in chimpanzees, gorillas, antelopes, and porcupines; but, like many zoonotic viruses, they believe the natural reservoir is fruit bats. Although it’s not known exactly how the child in Meliandou caught Ebola in the first place, before he fell ill, he was seen playing near a hollow tree where bats roost. Another human-
Evolution Challenges Efforts to Control Malaria
malaria A disease transmitted by mosquitoes that results from infection by a protozoan parasite of the genus Plasmodium; its life cycle uses hosts: mosquitoes and humans.

How does a parasitic disease like malaria show the complex relations between environment and infectious disease?
Diseases have evolved over millions of years to persist in species and ecosystems, and our efforts to eliminate them are often foiled by natural selection. One such disease is malaria, which is caused by a protozoan parasite carried by mosquitoes. Scientists have identified more than 100 species of malaria that infect birds, reptiles, and mammals. Six of these species are known to infect humans, primarily in tropical countries where malaria-
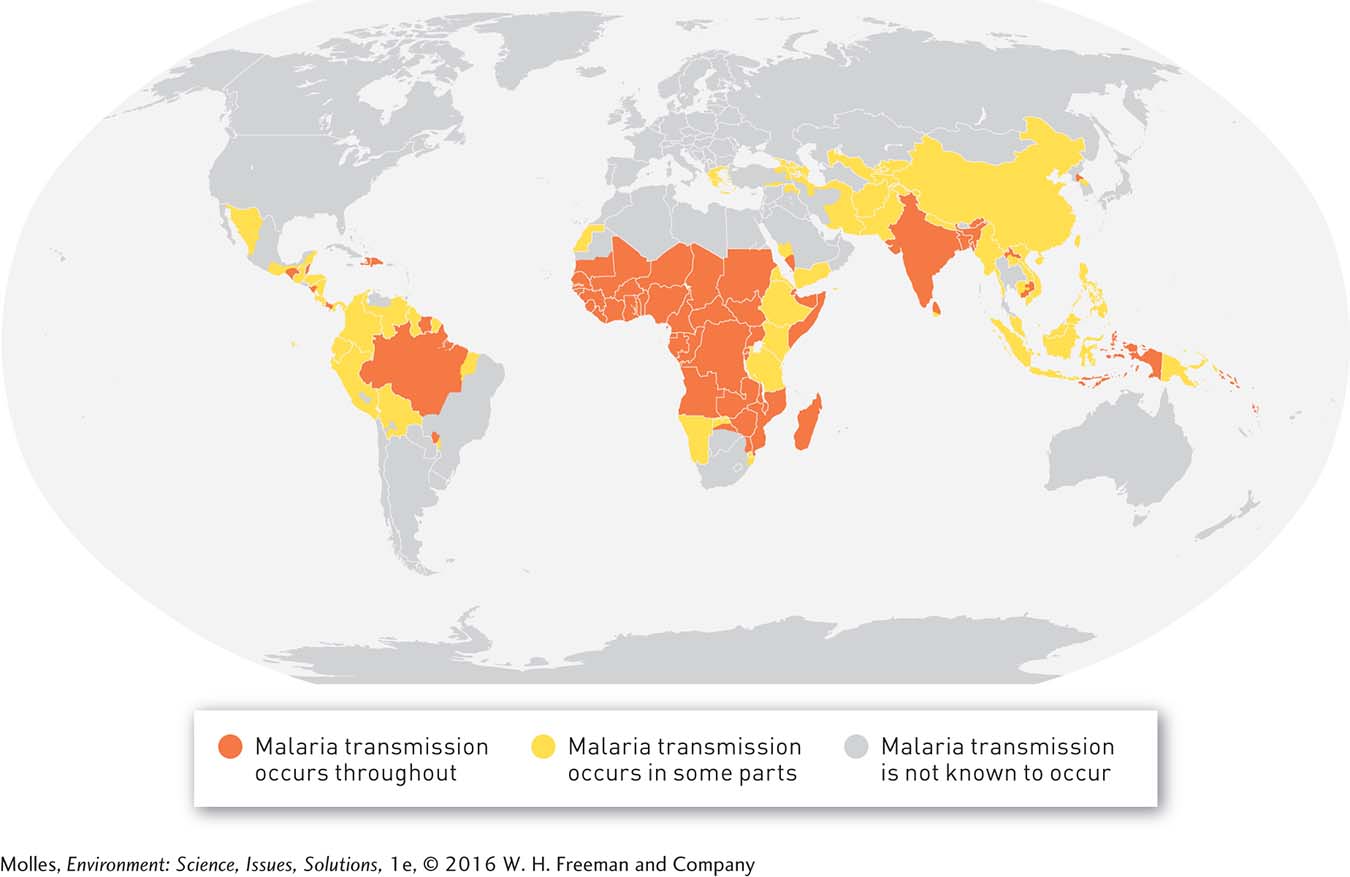
The best weapons we have against malaria after a person has already been infected are antimalarial drugs and pesticides that can reduce infection rates by reducing mosquito populations. However, both malaria parasites and mosquitoes are living organisms, and purely by chance some individuals within a population will be resistant to any new pesticide or antimalarial drug. When a pesticide or antimalarial drug is used, some of the resistant individuals will survive the treatment; those individuals will give rise to a new generation with greater resistance than their parents’ generation. This process repeats itself generation after generation, ultimately rendering the pesticide or antimalarial drug ineffective. Both these evolutionary processes have frustrated efforts to control malaria.
Quinine was an effective antimalarial drug during the 18th and 19th centuries, as European nations established colonies in tropical regions around the world. But by the mid-
345
Reports in a 2012 issue of the medical journal The Lancet further emphasized the point: “Antimalarial control efforts are vitally dependent on artemisinin combination treatments. Should these regimens fail, no other drugs are ready for deployment, and drug development efforts are not expected to yield new antimalarials until the end of this decade.”
If the parasite can’t be eliminated, is it possible to eliminate the mosquitoes that transmit the parasite? In the 1950s and 1960s, the WHO conducted an anti-
Think About It
How does a lack of education hinder efforts to stop the spread of zoonotic diseases such as Ebola?
How might malaria control programs be managed to reduce the likelihood that the parasite or vector will evolve resistance?
Why are environmental health scientists concerned about how global warming may affect efforts to control malaria?
11.3–11.6 Issues: Summary
Movement of toxic substances through the environment, persistence, bioaccumulation, and biomagnification add to the potential for harm to humans and other organisms. The use of chemicals such as endocrine disruptors, antibiotics, and pesticides can cause significant harm to the environment and to human health. Endocrine disruptors interfere with a variety of essential metabolic and developmental processes. Alterations of the normal development and function of sex organs are among their more conspicuous effects.
Human factors such as misuse and overuse of antibiotics can increase the spread of antibiotic-