Reduced Emissions and Decreased Acid Rain
Over the course of 20 years, the EPA’s Acid Rain Program was a success. By 2010 sulfur dioxide emissions were 12 million tons lower than in 1980, and emissions of nitrogen oxides were reduced by 4 million tons between 1995 and 2010 (Figure 13.30). One of the major contributors to these decreases was that electrical utilities switched from using high-sulfur coal from Appalachia to low-sulfur coal from the western United States, particularly from Wyoming’s Powder River Basin (see Figure 9.31, page 287). As predicted, acid rain also decreased (Figure 13.31). Precipitation in the eastern portion of the country became less acidic between 1994 and 2009 and acid rain (pH <5.3) was essentially eliminated from the central part of the country to the Pacific Coast.
TEMPORAL TRENDS IN EMISSIONS OF POLLUTANTS THAT CAUSE ACID RAIN
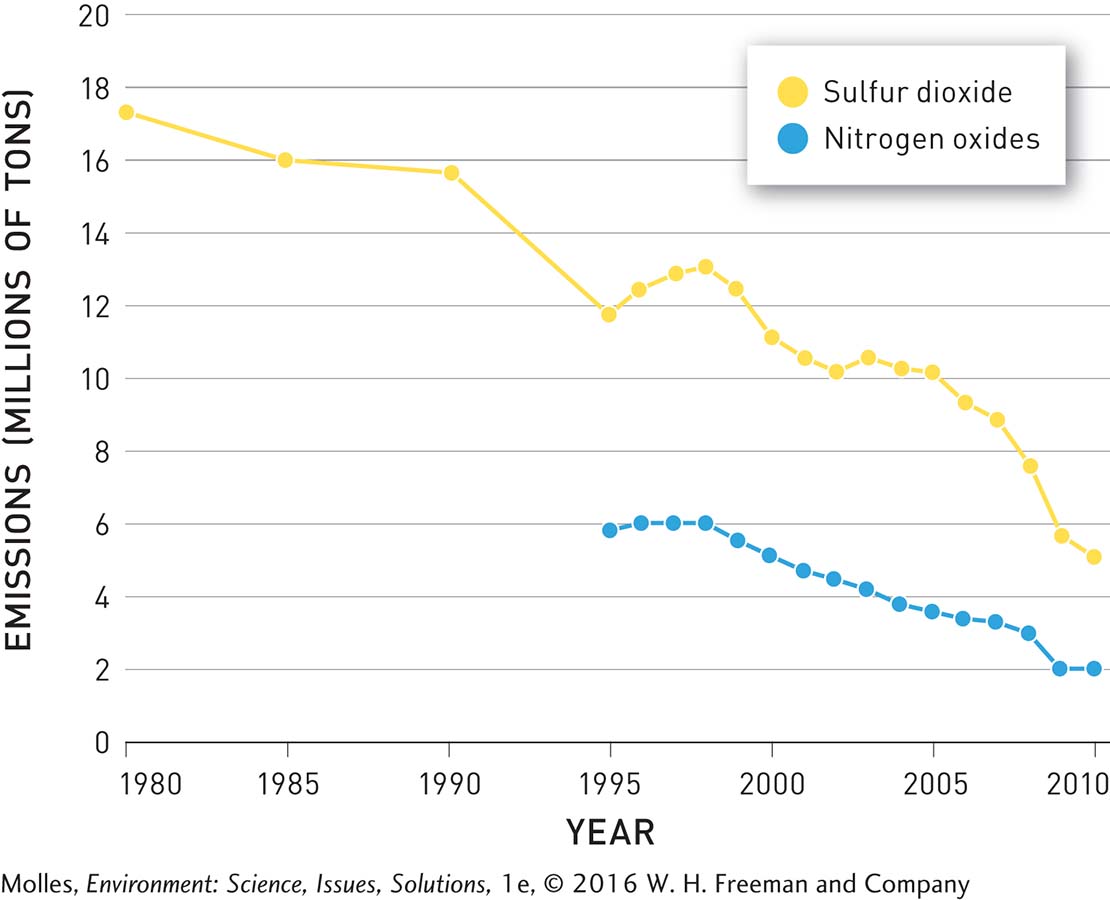
FIGURE 13.30 Emissions of sulfur dioxide (SO2) and nitrogen oxides (NOx) from electrical power plants in the United States decreased significantly following regulation by the Clean Air Act. (Data from the EPA, 2015a)
pH OF PRECIPITATION ACROSS THE UNITED STATES IN 1994 AND 2009
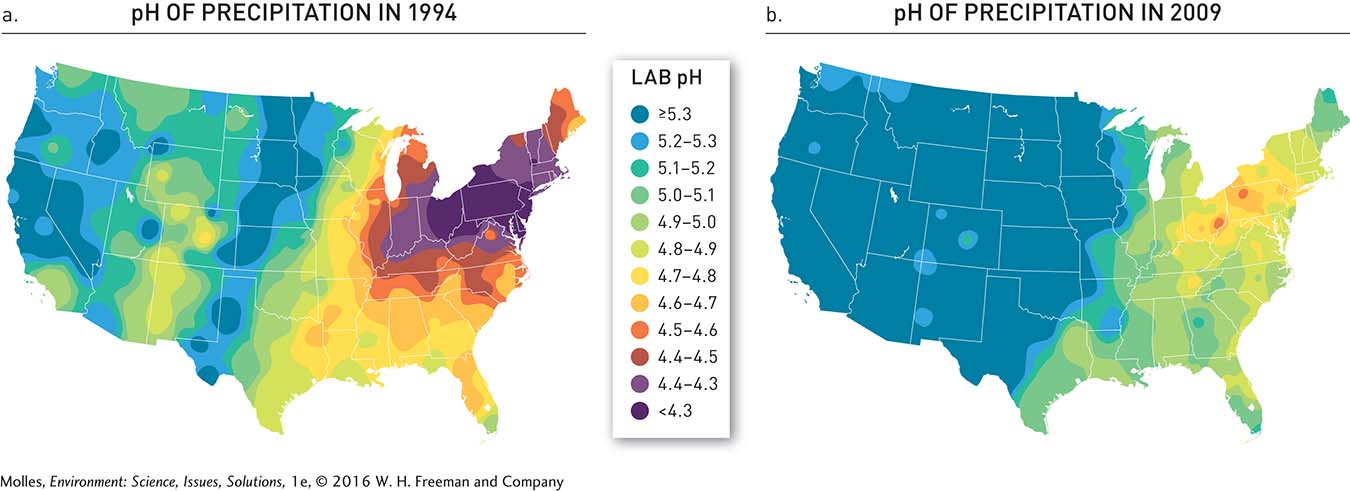
FIGURE 13.31 (a) In 1994 precipitation was highly acidified (pH <5.3) in the eastern half of the country, especially in the upper Midwest and Northeast. (b) By 2009, however, acid rain control programs resulted in substantial reductions in the acidity of rain across the country. (MAPs from National Atmospheric Deposition Program, http://nadp.sws.uiuc.edu/)
Recovery of Aquatic Ecosystems
With reduced acid rain, lakes and streams that had been acidified during the 1970s and 1980s began to recover. Consider an example from the Adirondack region of New York State. Prior to the start of the Acid Rain Program, 284 lakes out of nearly 2,000 being monitored there were highly acidified. Within 2 to 3 years, that number dropped by 32% to 192 (Figure 13.32). By 2007, 132 of the originally acidified lakes had recovered. Streams in the Adirondack region are also recovering, although at a slightly slower rate.
RECOVERY OF HIGHLY ACIDIFIED LAKES IN THE ADIRONDACK REGION OF NEW YORK
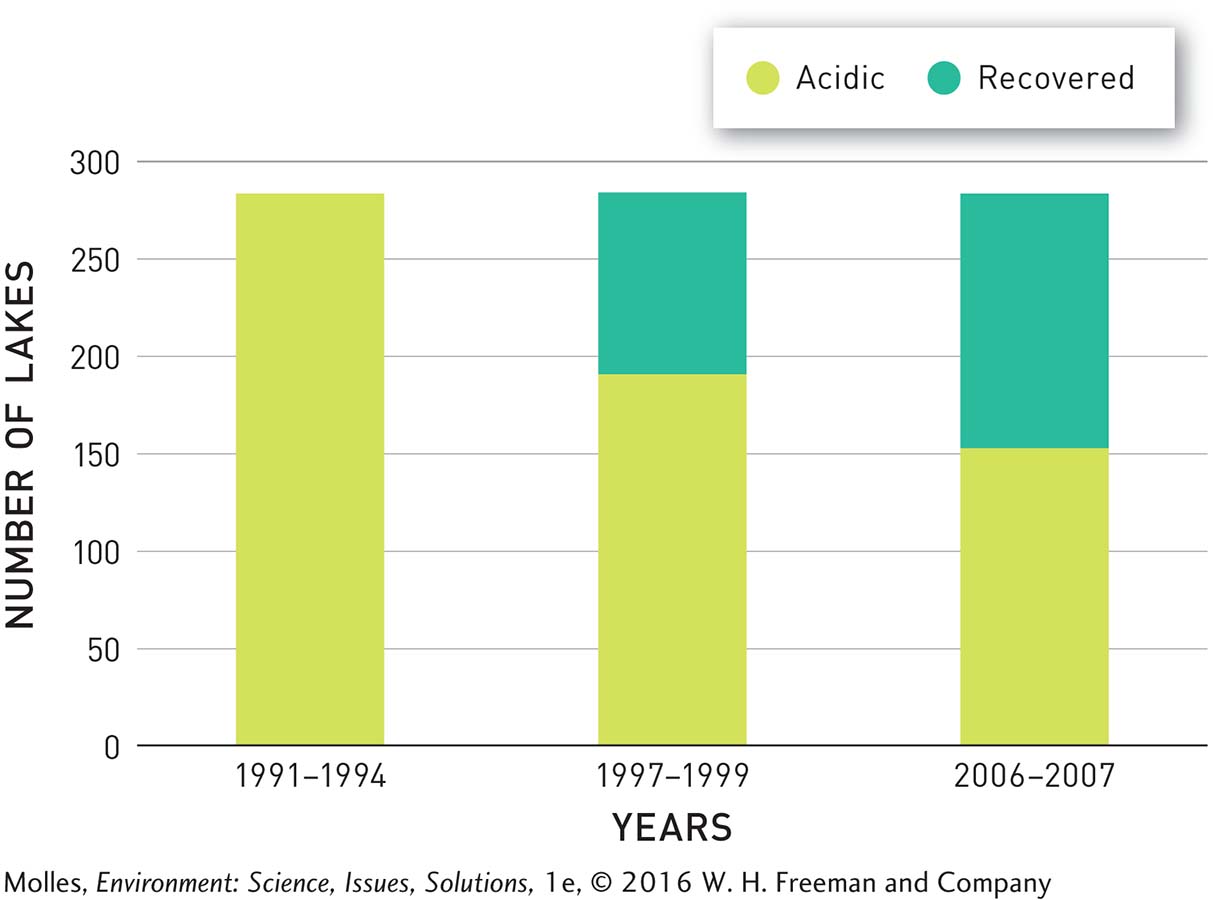
FIGURE 13.32 At the beginning of the EPA’s Acid Rain Program in 1995, 284 lakes in the Adirondack region were highly acidified. By 2007, 132 of those lakes, or 46%, had recovered in response to decreased acid rain over the region. (Data from Waller et al., 2012)
Up near Sudbury, Clearwater Lake is also showing remarkable recovery. Beginning in 1972, pollution controls decreased the SO2 emissions of the smelters in the Sudbury areas by more than 90%. In response, the pH of Clearwater Lake, and of other lakes in the area, increased (Figure 13.33). After 1999 the pH in Clearwater exceeded 6.0, a threshold allowing for full recovery of the ecosystem. The concentrations of heavy metals, such as nickel and copper, have also decreased following pollution control (Figure 13.34).
TRENDS IN SO2 EMISSIONS AND LAKE pH
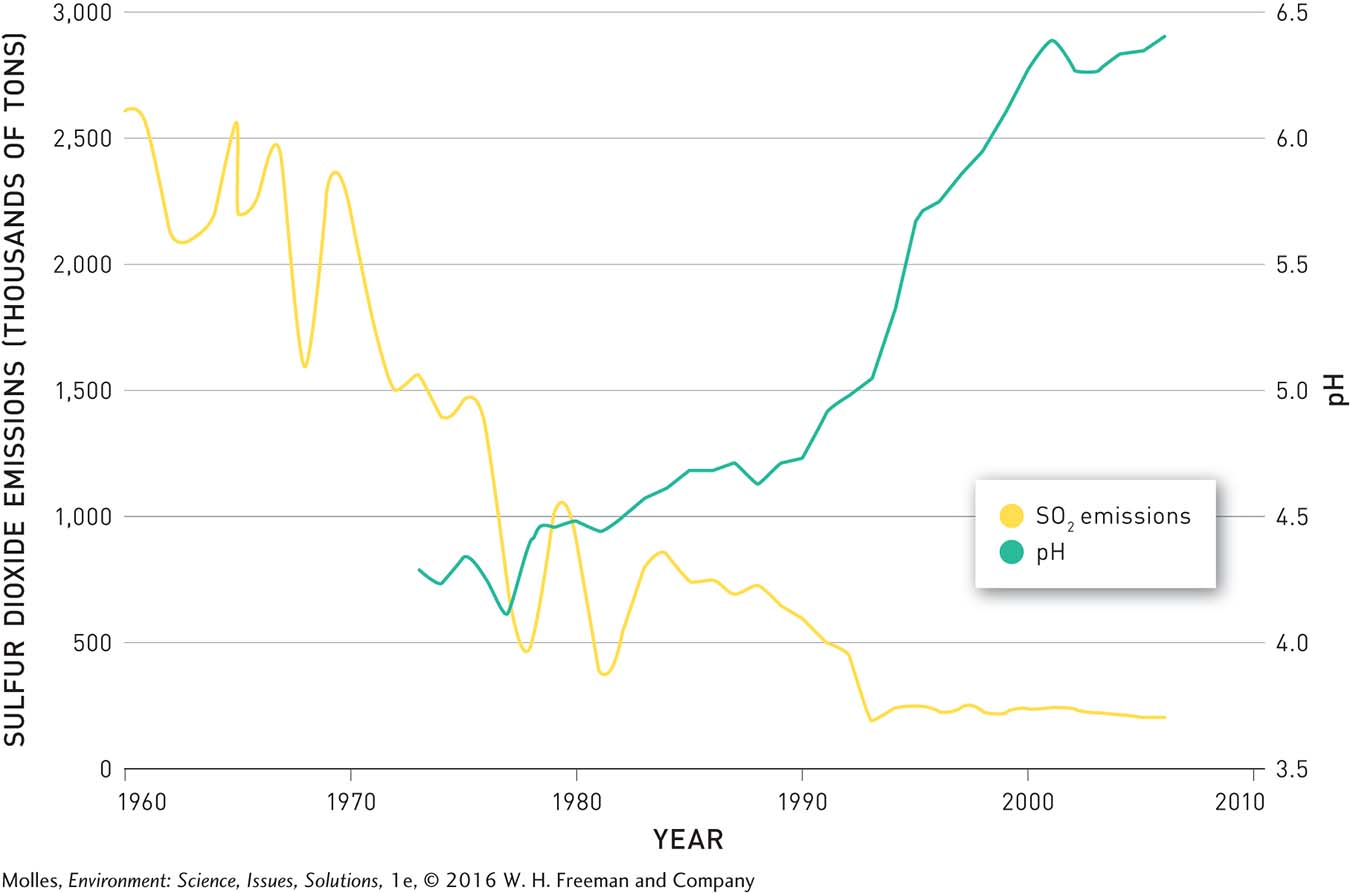
FIGURE 13.33 Total annual sulfur dioxide emissions from the smelters near Sudbury, Ontario, in thousands of metric tons and pH in Clearwater Lake, located 13 kilometers from Sudbury. (Data from Keller, 2009)

What role do you think the flexibility allowed to utility companies by the EPA played in the successful reduction of SO2 and NOx emissions? Explain.
TRENDS IN NICKEL AND COPPER CONCENTRATIONS IN CLEARWATER LAKE NEAR SUDBURY, ONTARIO
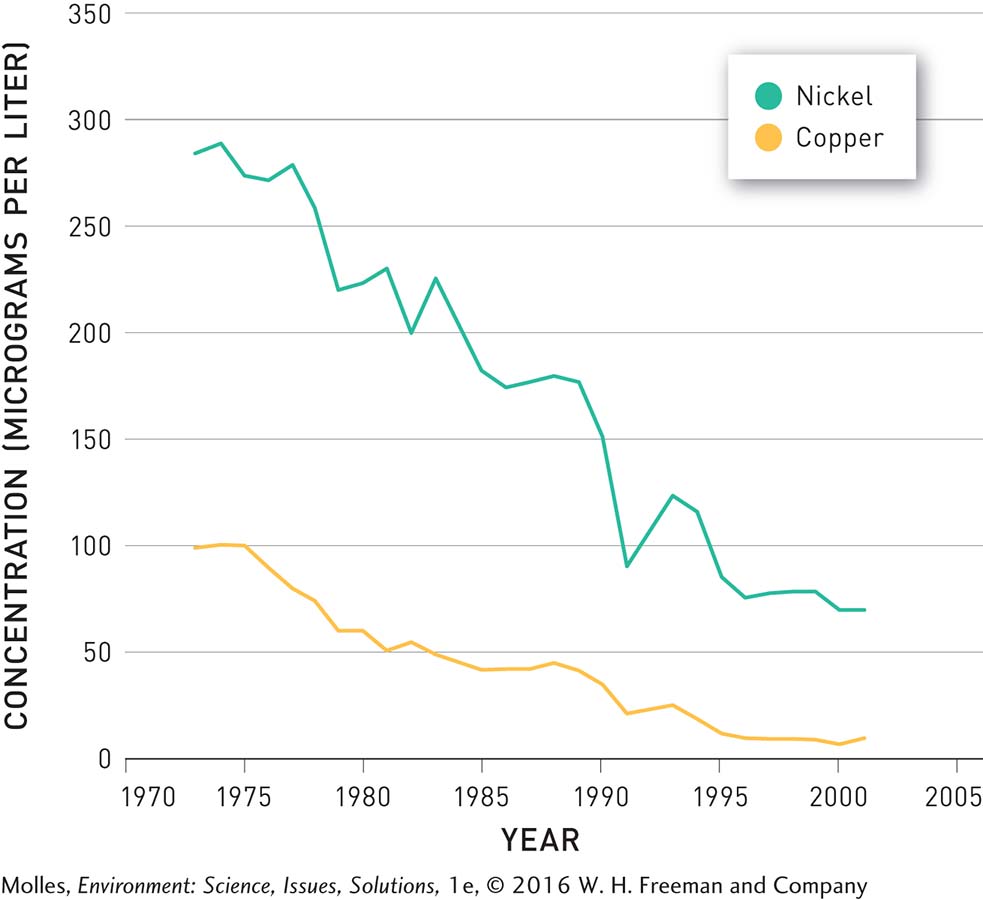
FIGURE 13.34 Over the course of nearly three decades, following reduced pollution from the metal smelters in the area, the concentrations of both nickel and copper in Clearwater Lake decreased significantly. (Data from Girard et al., 2006)

Why might non-native species have an advantage over native species in the face of a disturbance, such as acid rain?
However, recovery of the biological community has been uneven. The phytoplankton and zooplankton of many lakes have bounced back, but neither invertebrates that live at the bottom of the lake nor fish are back to natural levels yet. Factors that may retard recovery in certain areas include competition with established acid-tolerant species. Once a community of organisms is established, in this case of acid-tolerant species, they can persist in an ecosystem and compete with individuals of less tolerant species when physical conditions change. Predation by high numbers of invertebrate predators, which have been released from control by plankton-feeding fish eliminated by acid rain, can also reduce the chance of successful colonization by small organisms. In addition, limited dispersal slows colonization by some organisms. Another problem has been high heavy metal concentrations and reduced concentrations of calcium, Ca2+.
Recovery of Terrestrial Ecosystems
Restoring the forests that once covered the Sudbury landscape is a huge challenge—much greater than restoring a forest following logging or fire. The main reason for the difficulty is that the damage done to these ecosystems has seriously impaired the very foundation of terrestrial ecosystems: their soils. Succession in such circumstances takes a very long time unless helped along by active restoration methods. One of the main treatments used at restoration sites in the most disturbed areas has been spreading crushed limestone on the soils. Limestone adds the critical Ca2+ ions stripped from the soils by acid rain and can neutralize acidic soils, resulting in higher soil pH. Liming at the restoration sites has raised average soil pH to 6.3, compared with 4.3 at unrestored sites. Some sites were limed and planted with a single species of pine. Others were limed, fertilized, and planted with three to five tree species.
As a result of active restoration, the number of plant species at the restored sites averaged more than 3 times greater, compared with unrestored sites in the most disturbed zone (Figure 13.35). The number of plant species at the most species-rich restoration site was comparable to that at the low disturbance site, approximately 36 kilometers from the decommissioned smelter. However, approximately 30% of the plant species at restored sites were invasive non-native species, which have high dispersal rates and had colonized the restoration sites on their own. Meanwhile, all plant species at unrestored, control sites were native. Researchers suggest that it may take decades or even centuries for the plant communities at the restored sites to return to a composition close to the original community before disturbance.
RESULTS OF TERRESTRIAL RESTORATION AT SUDBURY, ONTARIO
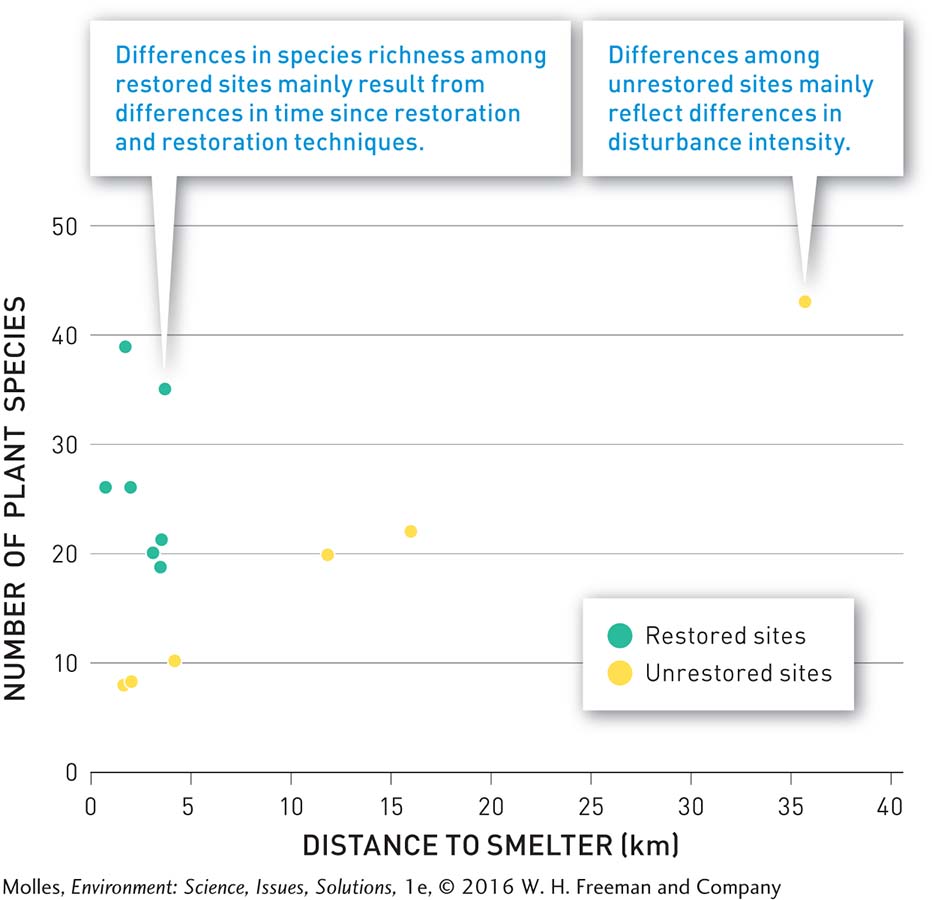
FIGURE 13.35 Decades of natural recovery and active restoration near a decommissioned smelter have shown that active restoration speeds the development of species-rich plant communities in the most disturbed sites. (Data from Rayfield et al., 2005)
Think About It
What do the successful reductions in acid rain and recovery of some ecosystems indicate about the scientific understanding of the causes and effects of acid rain?
Why might the ecosystems impacted by acid rain never return to their state prior to acid rain impacts, especially in terms of their species composition?
What can working toward restoration of damaged ecosystems teach us about the ecology of these systems?







