13.1 Industry releases pollutants
13.1–
388

pollution Contamination of the environment, generally of air, water, or soil, by substances or conditions (e.g., noise, light) at levels harmful to living organisms; generally a result of human activity but may result from natural processes (e.g., wildfires, volcanic eruptions).
pollutant A substance (e.g., oil, pesticides) or physical condition (e.g., excessive noise) harmful to living organisms that contaminates air, water, or soil.

Is there a connection between population growth and pollution? Explain.
Pollution is defined as contamination or physical alteration of the environment at levels harmful to living organisms. The factors causing pollution, pollutants, include chemical substances, such as oil or pesticides, or altered physical conditions, such as excessive noise or light (Figure 13.1). Natural events, such as wildfires or volcanic eruptions, can cause pollution, but at this point in the history of Earth, pollution mainly results from human activity.

Concerns about environmental contamination began long ago. As we saw in Chapter 1, Benjamin Franklin and other residents of 18th-
Point Versus Nonpoint Sources of Pollution
point sources of pollution Clear-
nonpoint sources of pollution Diffuse, and sometimes mobile, sources of pollution (e.g., runoff from an industrial, municipal, or agricultural landscape or the exhaust from automobiles).

Why might secondary pollutants present greater challenges to management than primary pollutants?
Pollutants are released into the environment through one of two avenues: from point sources or from nonpoint sources (Figure 13.2). The smokestack of a factory or a sewer pipe discharging pollutants into the environment from a clearly definable place is called a point source of pollution. Nonpoint sources of pollution introduce pollutants from scattered locations or may move around in the environment. Examples of nonpoint sources include automobile exhaust or polluted water draining from city streets or pesticide-

389
Pollutant Persistence
Some pollutants break down rapidly when released into the environment, whereas others persist for months, years, centuries, or millennia. Bacteria and fungi will quickly metabolize simple organic compounds, such as sugars and amino acids. More complex organic compounds, such as the cellulose component of wood, though biodegradable (see Chapter 12, page 366), will decompose much more slowly. Decomposition of such compounds will occur over a period of months or years, depending on environmental conditions, such as oxygen concentration or temperature. In contrast, atmospheric scientists estimate that some fraction of the carbon dioxide released by fossil fuel combustion will remain in the atmosphere for thousands of years before being taken up by natural processes, such as photosynthesis. Meanwhile, heavy metal pollutants do not break down; they simply persist.
Primary and Secondary Pollutants
primary pollutant A substance that is harmful when released into the environment (e.g., carbon monoxide, crude oil).
secondary pollutant A pollutant formed from the chemical reactions between other pollutants (e.g., ozone in the lower atmosphere).
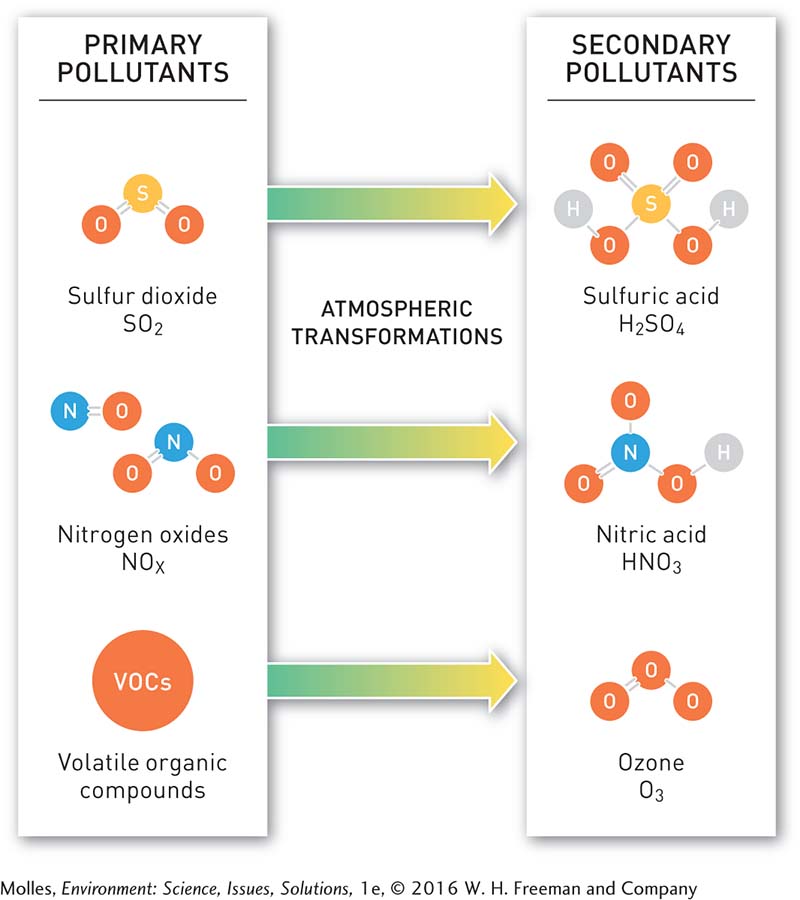
Substances that are harmful when released into the environment are called primary pollutants. Primary pollutants in air include carbon monoxide, lead, nitrogen oxides, particulate matter, and sulfur dioxide. Meanwhile, some primary air pollutants react chemically to form secondary pollutants. For example, ozone (O3) is formed through the reaction of oxides of nitrogen (NOx) with volatile organic compounds, or VOCs—
acid A substance that releases hydrogen ions upon dissociation when dissolved in water, resulting in reduced pH of the solution; acids neutralize bases.
pH An indicator of the relative hydrogen ion concentration of a solution. A pH of 7 indicates a neutral solution; a pH of less than 7 is acidic (elevated hydrogen ion concentration); a pH greater than 7 is basic (reduced hydrogen ion concentration).
Another notable example of the formation of a secondary pollutant occurs when SO2 and NOx undergo reactions in the atmosphere that produce strong acids: SO2 reacts to form sulfuric acid and NOx reacts to form nitric acid (Figure 13.3). An acid is a substance that releases hydrogen ions (H+) upon dissociation when dissolved in water, resulting in reduced pH, an indicator of relative hydrogen ion concentration, of the solution. A pH of 7 indicates a neutral solution, while a pH of less than 7 is acidic (elevated hydrogen ion concentration), and a pH greater than 7 is alkaline (reduced hydrogen ion concentration). An important feature of the pH scale is that the units are base-
Think About It
How might degree of persistence affect management of different pollutants?
390
Why is a reduction in the pH of lake water by just 2 units, from pH = 7 to pH = 5, a significant change in the environment?