14.2 Scientists began building the basis for understanding the greenhouse effect more than 200 years ago
The fast-paced study of climate change today has its roots in some landmark scientific discoveries dating back to the 19th century. After 35 years of playing the oboe, violin, and harpsichord, the German-born composer Fredrick William Herschel began experimenting with a new instrument: the telescope. On February 11, 1800, he was testing out colored filters to observe Sun spots and noticed that filters of different colors seemed to pass different amounts of heat. Observing these differences, he decided to measure how much heat was carried by various colors of light. To do so, Herschel used a prism to break up the solar spectrum into its component wavelengths and then compared the temperatures recorded by thermometers exposed to each color of light with the temperature recorded by two thermometers outside the light beam (Figure 14.5). To his surprise, he found that the highest temperatures of all occurred just beyond the red portion of the spectrum, where there was no visible light! Herschel found that these “calorific rays,” as he referred to them, were absorbed, reflected, and transmitted just like visible light. He had discovered infrared radiation.
DISCOVERING INFRARED LIGHT
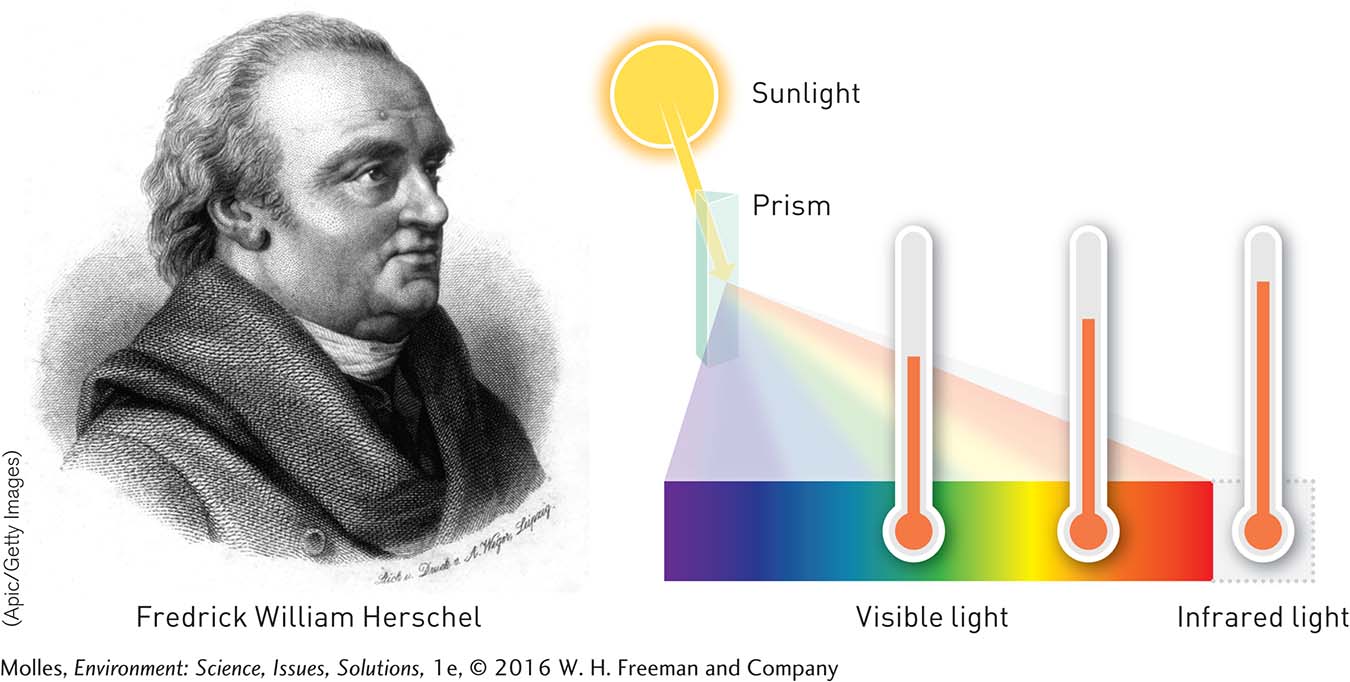
FIGURE 14.5 Using an ingenious device of his own design, Fredrick William Herschel accidentally discovered the existence of invisible infrared light in 1800 while measuring the heat content of light bands within the visible spectrum. In the part of his experiments shown here, one of the thermometers is positioned just beyond the red portion of the spectrum, where Herschel measured higher temperatures than in any of the visible parts of the solar spectrum.
(Apic/Getty Images)
Not long afterward, the French mathematician Jean-Baptiste Fourier recognized the significance of infrared light to Earth’s climate. In 1827 Fourier proposed that a planet gained energy primarily from the Sun and continued to increase in temperature until its rate of energy gain was equaled by its rate of energy loss in the form of infrared light. He also suggested that Earth’s atmosphere was analogous to a pane of glass, which readily transmits visible light but is much less transparent to infrared light. Although Fourier made a number of errors, he established the central concepts of the greenhouse effect that are still in use today.
One thing missing from Fourier’s understanding of the greenhouse effect was the significance of various gases in the atmosphere. By passing infrared light through a gas-filled tube, the British physicist John Tyndall was able to estimate how much infrared light a particular gas absorbed (Figure 14.6). He found that oxygen and nitrogen were largely transparent to infrared light. However, trace gases in the atmosphere—including water vapor, carbon dioxide, and nitrous oxide—were strong absorbers of infrared light. Without these trace gases, Tyndall asserted that Earth would transform into a frozen world.
TYNDALL’S INSTRUMENT FOR MEASURING ABSORPTION OF INFRARED LIGHT BY TRACE GASES
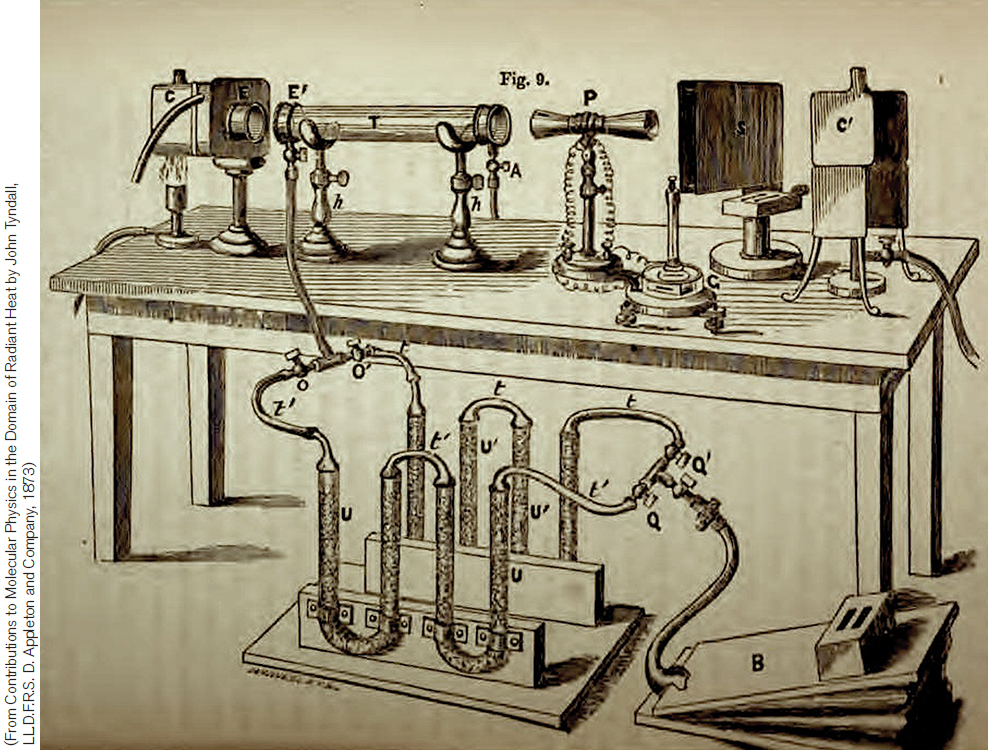
FIGURE 14.6 In 1861 John Tyndall published the results of his studies demonstrating that a number of trace atmospheric gases absorb infrared light, thereby identifying the mechanism for the warming of Earth’s atmosphere proposed a quarter-century earlier. In his tests, Tyndall would fill a tube in the instrument with a particular atmospheric gas, such as carbon dioxide. Then, by passing infrared light through the tube and measuring the amount absorbed, he could determine the potential contribution of that gas to the greenhouse effect. (From Tyndall, 1861)
(From Contributions to Molecular Physics in the Domain of Radiant Heat by John Tyndall, LL.D.F.R.S. D. Appleton and Company, 1873)
Indeed, geologic research at that time was starting to point toward the existence of such a frozen world in the past. During the last Ice Age, Northern Europe and North America were covered by extensive glaciers. At other times, the climate had been much warmer. What was responsible for the wide variation in climate? The Swedish chemist and Nobel Laureate Svante Arrhenius proposed that it resulted from processes that produced variation in atmospheric carbon dioxide. For instance, volcanic eruptions introduced carbon dioxide into the atmosphere, whereas the formation of carbonate rocks and coal reduced it. By the early 20th century, Arrhenius pointed out, carbon dioxide emissions from the burning of coal rivaled emissions from volcanoes, and increased burning of coal could lead to future global warming.
He predicted that a further doubling of atmospheric carbon dioxide would raise global temperatures 5° to 6°C, while a halving of those levels would decrease global temperatures by 4° to 5°C. Arrhenius was generally correct about the impact of carbon dioxide and other atmospheric gases on global temperatures. However, he had no satisfactory explanation for how cycling of the climate between ice ages and warmer periods is produced. We now know that these climate cycles are triggered by variation in the tilt of Earth’s axis and the shape of its orbit around the Sun.
Think About It
Imagine looking at the world with an infrared camera. What objects would glow most brightly?
Can you find examples of volcanoes changing the climate in the past?

