2.2 Energy makes matter move
In a forest, a hawk pursues a fleeing sparrow through the sun-
Energy and Work
energy The capacity to do work. See work.
work A description of the transfer of energy; the work done on an object by a force is determined by the amount of force times the distance the object moves in the direction of the force. See energy.
Describing a person as having lots of energy means that he or she is vigorous or lively. Physicists use the term in a much more precise way, defining energy as the capacity to do work. Work is the product of the amount of force applied to an object and the distance the object is moved in the direction of the force. Certainly, a vigorous person moving his or her arms around will be performing a lot of work! Similarly, the amount of work required to put a book on a shelf depends on the weight of the book and the height of the bookshelf (Figure 2.4). Consequently, you burn more calories (i.e., energy) lifting a heavy book to a higher shelf than you would expend to lift a lighter book to a lower shelf. The same is true when it comes to a jet airliner filled with passengers and luggage ascending from sea level to an altitude of 10,000 meters (33,000 feet). That takes a huge amount of work and, consequently, requires substantial energy in the form of jet fuel.
37

Forms of Energy

Use the concept of energy to explain how reading the words on this page and thinking about the concepts discussed qualify as work.
potential energy The amount of energy an object has due to the configuration of its parts (e.g., a loaded spring), its chemical makeup, or its position in a force field (e.g., Earth’s gravitational field).
chemical energy A form of potential energy; energy stored in the bonds of molecules, such as sugars, fats, or methane.
kinetic energy The energy of a moving object, which is equal to one-
gravitational potential energy The amount of potential energy an object contains due to its mass and height above a reference point, such as Earth’s surface.
The energy to perform work comes in many different forms. Think about a battery in your desk drawer. It’s not performing any work, but it has the capacity to do so, which we call potential energy. Likewise, the source of the energy we use to lift our arm and place a book on a shelf is stored as chemical energy in the bonds of molecules, such as sugars and fats. As the chemical energy fuels the contraction of your muscles, it is converted into kinetic energy, the energy of movement. When our book is placed on a shelf, the energy of placing it there is transferred to the book, as gravitational potential energy, which is a consequence of its position above ground. This potential energy would be released as kinetic energy (movement) if the book were to fall off the shelf and crash to the floor.
thermal (heat) energy A form of kinetic energy due to molecular motion in a mass of a substance, such as a mass of steam.
As fuel is processed, whether in the cells of your body while you are exercising or in a jet engine during takeoff, another form of kinetic energy is released: Thermal (or heat) energy results from the motion of molecules in a substance and therefore is a form of kinetic energy. The faster the molecules move, the hotter the substance becomes. When it’s cold, our bodies use heat released through shivering to stay warm. Steam locomotives move trains down the track by harnessing the thermal energy in steam to move the pistons that turn their wheels.
radiant energy The energy of electromagnetic radiation, including visible light, infrared light, ultraviolet light, microwaves, radio waves, or X-

How are a compressed spring and a sugar molecule similar?
Sunlight is made up of radiant energy, which is the energy of electromagnetic radiation, including visible light, infrared light, and ultraviolet light, as well as microwaves, radio waves, and X-

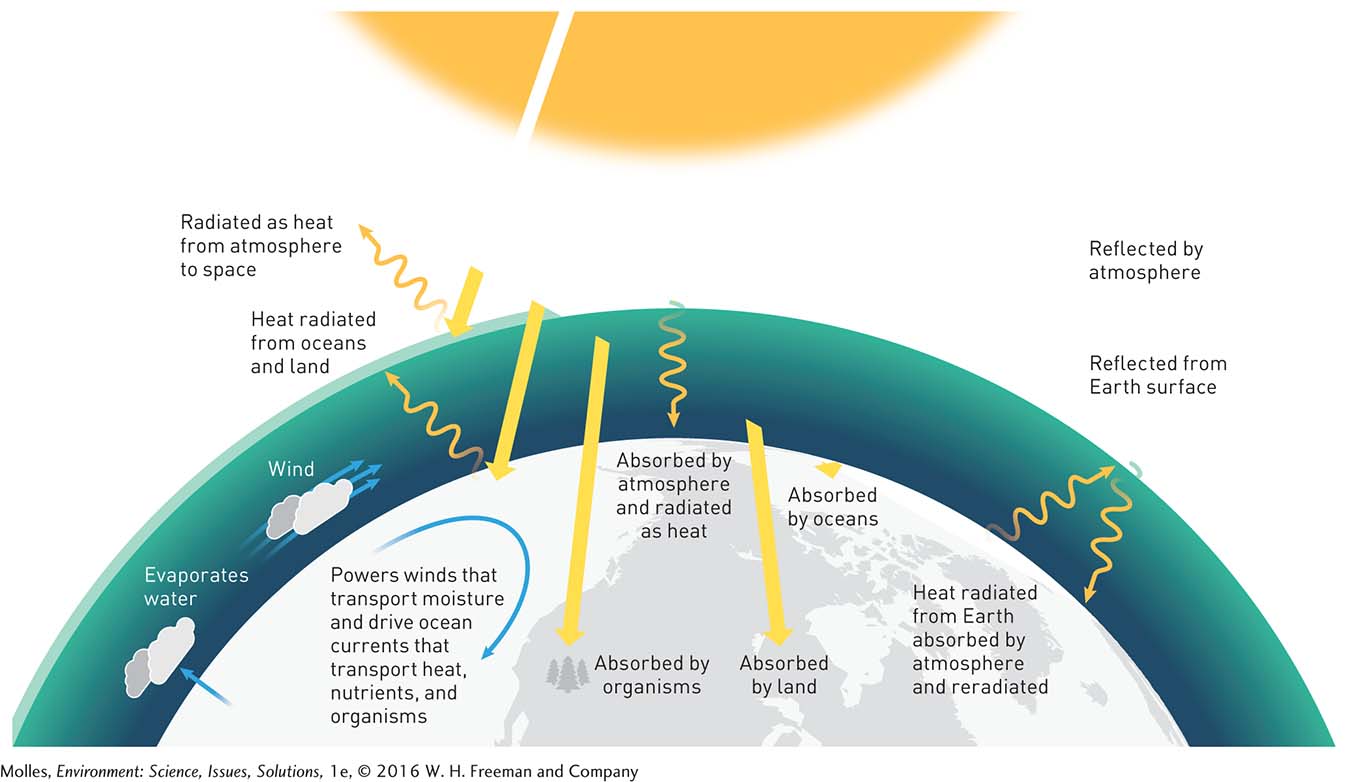
Energy in all its forms is in action all around us. For example, solar energy drives the movement of atmospheric gases that we call wind, another example of kinetic energy. The heat radiating from a sidewalk on a hot day is an example of radiant energy being released by the sidewalk, which has been heated by sunlight. Some solar energy is converted to heat energy stored in the surface water of lakes and oceans, causing some water to evaporate, which transforms liquid water to gaseous water vapor. This water vapor may eventually condense to form clouds, one of the fundamental processes in the hydrologic cycle (see Figure 6.4, page 160). All the forms of energy operating at scales ranging from placing a book on a shelf to global atmospheric processes follow a set of strict physical laws.
The Laws of Thermodynamics
first law of thermodynamics
A physical law concerned with the conservation of energy: Though one form of energy may be transformed to other forms, the total amount of energy in a system plus its surroundings is conserved; that is, the total amount of energy remains the same. See second law of thermodynamics.
The first law of thermodynamics states that when one form of energy is transformed to other forms, the total amount of energy in a system and its surroundings remains the same. For instance, if we were to measure incoming solar energy and then add up the related energy on Earth and its surroundings, including the energy of wind or the energy absorbed by trees and stored in our own bodies, we would find that they are the same (Figure 2.6). We call this the conservation of energy.
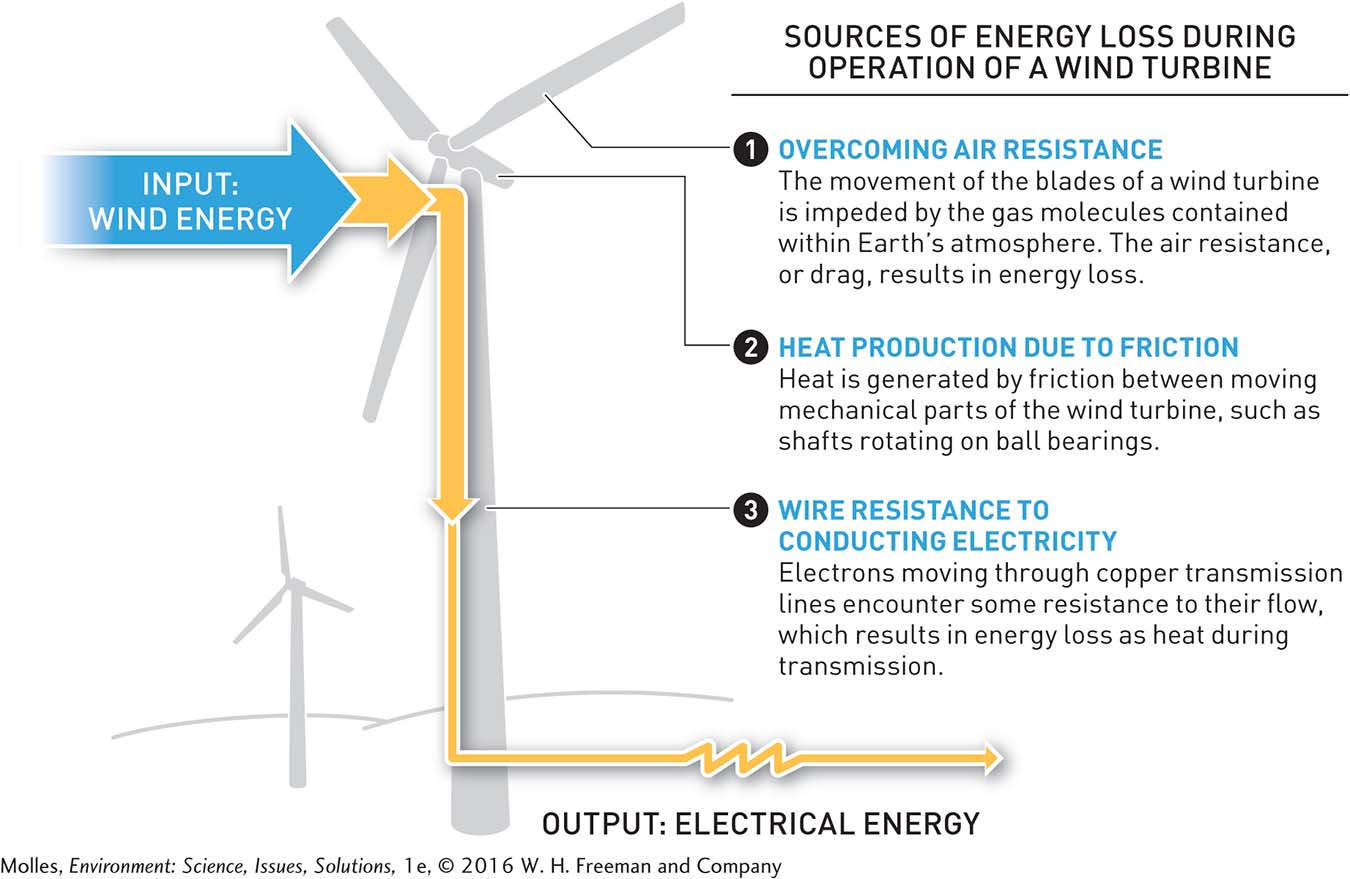
However, if you were to actually measure the kinetic energy of the wind turning the blades of a wind turbine and then the electricity produced by the wind turbine, you would find that there is less electrical energy than wind energy (Figure 2.7). How can this be? The first law of thermodynamics states that the total amount of energy in the system plus the surroundings must remain the same. Some of the difference can be accounted for by the production of heat by friction between the moving and stationary parts of the turbine mechanism. If you were to touch the wind turbine, you would find that it is warm.
38
second law of thermodynamics With each energy transformation, or transfer, the amount of energy in a system available to do work decreases. In other words, the quality of the energy declines with each energy transfer or transformation. See first law of thermodynamics.
entropy A measure of the amount of disorder in a system.
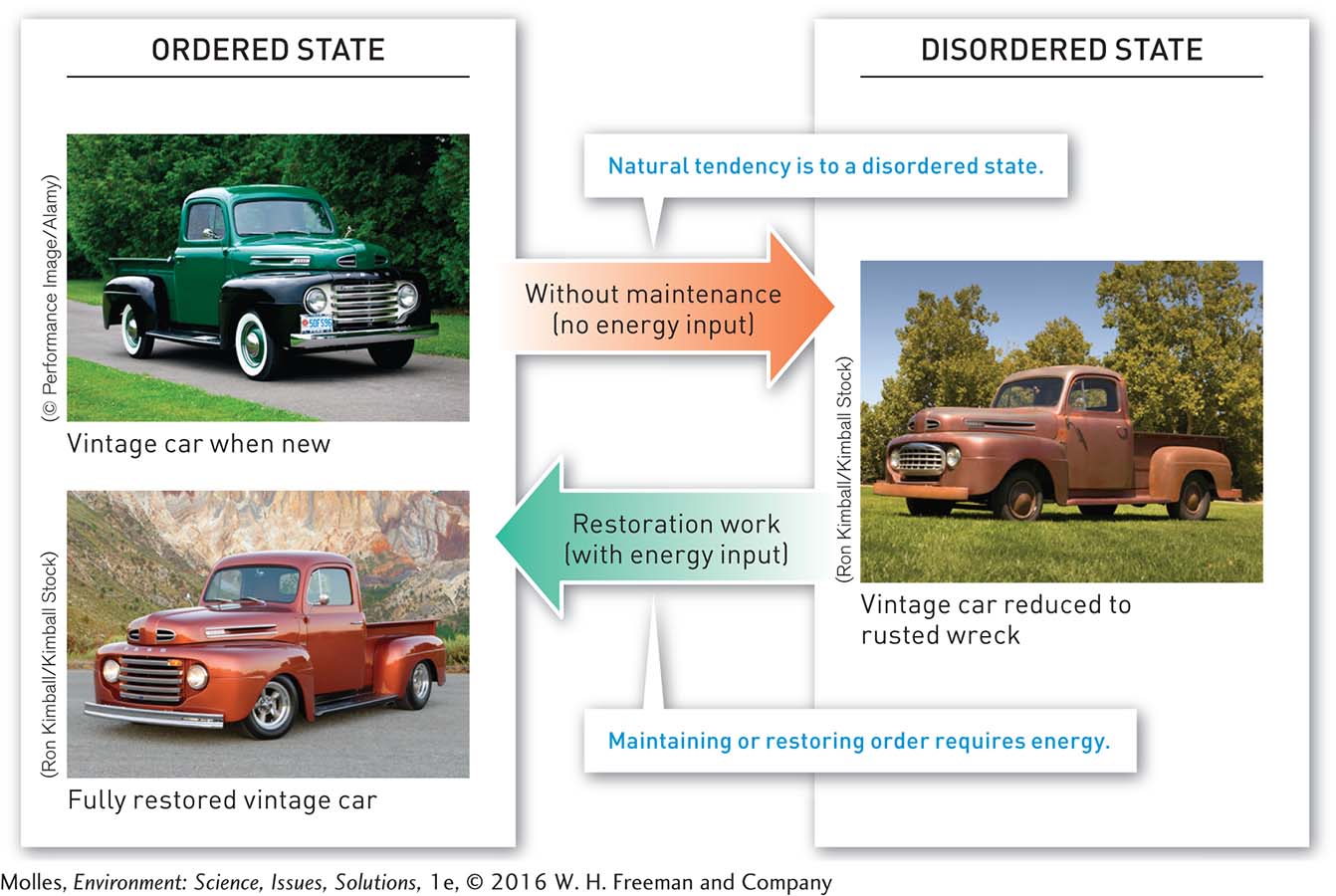
This brings us to the second law of thermodynamics, which states that with each energy transformation, such as the conversion of wind energy to electrical energy by a wind turbine, the amount of energy available to do work decreases because of the production of unusable heat energy. The second law also states that over time, entropy, or the amount of disorder in a system, increases. And so maintaining order in a system—
39
Think About It
As an engineer, what design features could you target to improve the energy efficiency of wind turbines?
Why is it impossible to build a mechanical device that is 100% efficient at converting wind energy into electrical energy?
Why is it impossible to engineer a mechanical system, such as an automobile, capable of being operated indefinitely with no physical maintenance?