6.9 Desalination taps Earth’s largest reservoir of water
seawater Ocean water and the water of seas, such as the Caribbean and Mediterranean seas. The salinity of seawater averages about 34,000 mg/l (34 g/l), but ranges from 30,000 to about 40,000 mg/l (30 to 40 g/l).
desalination The process of removing salts from seawater or brackish water to form freshwater.
It’s a frustrating fact of nature that humans cannot survive on seawater. Although salt is an essential mineral, the problem is that seawater is approximately 3 times as salty as our blood, and drinking it would quickly overwhelm our kidneys and lead to death. While seawater may have 35,000 milligrams per liter of salt, drinking water should contain less than 500 milligrams per liter (Figure 6.30). Desalination, the process of turning saltwater into freshwater, is one solution to finding drinking water where natural sources are scarce. In 2012 there were more than 16,000 desalination plants in operation, generating over 28 km3 of freshwater per year. That’s only 1% of freshwater used by humans from all sources, but the small flow of freshwater that it does produce is critical for sustaining some human communities.
DESALINATION OF SEAWATER AND BRACKISH WATER REQUIRES ENERGY
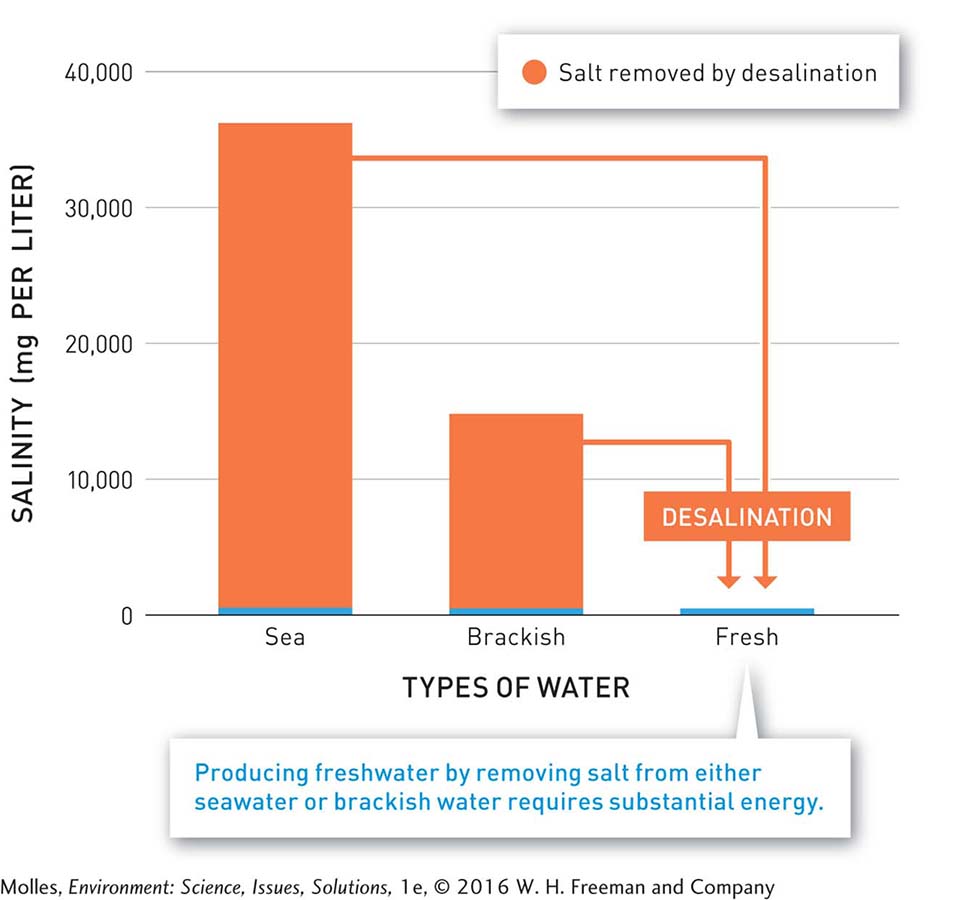
FIGURE 6.30 The red area of each bar shows the amount of salt that must be removed to convert (average) seawater or (median) brackish water to freshwater with salinity less than 500 milligrams per liter. (Data from Art, 1993)
distillation A desalination process that uses heat to evaporate water from seawater or brackish water and condenses the resulting salt-free water vapor to produce freshwater.
brackish water Natural waters with a salt content intermediate between freshwater and seawater, commonly occurring near the mouths of rivers where freshwater and seawater mix.
The earliest approach to desalination involved distillation, a process that uses heat to evaporate water from seawater or brackish water (natural waters with a salt content intermediate between freshwater and seawater) and then condenses (and captures) the resulting salt-free water vapor. The outputs from the distillation process are freshwater and concentrated salt brine (Figure 6.31). In 2012 distillation accounted for approximately 15% of water produced by desalination worldwide. Because it requires a lot of energy, this approach to desalination is generally expensive. However, various engineering techniques have improved the efficiency of the process a great deal.
DESALINATION BY DISTILLATION: EVAPORATION AND CONDENSATION OF WATER
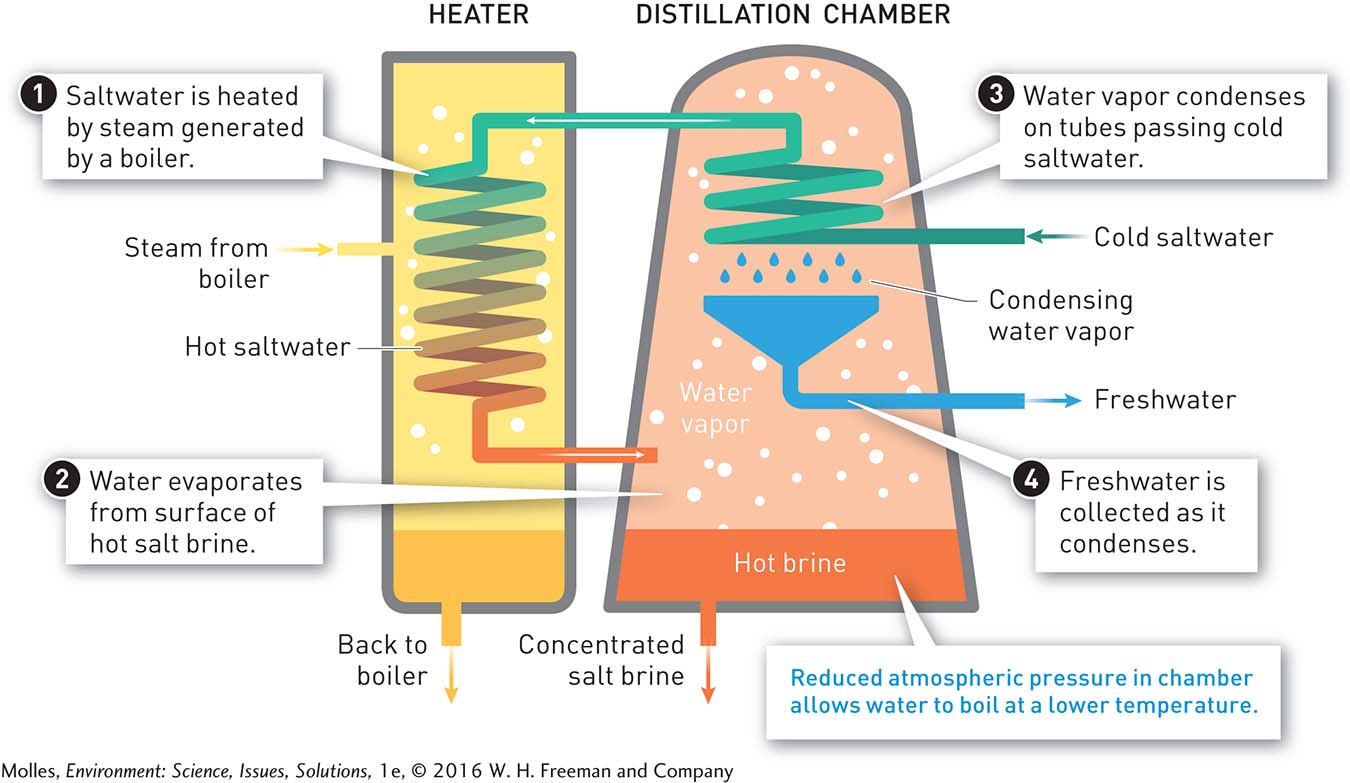
FIGURE 6.31 Distillation is the oldest method of desalination. The basic technology is well developed and dependable. Its major shortcoming is that the process requires a lot of energy. Lowering the atmospheric pressure in distillation chambers reduces energy costs for distillation. To increase water yield, several distillation chambers may be connected in series, each with a lower atmospheric pressure.
cogeneration Generally refers to the use of a single source of energy for multiple purposes.
For example, it is possible to use the heat produced as a by-product of electrical generation as a source of heat energy for desalination. This approach is called cogeneration, a term generally referring to the use of a single source of energy for multiple purposes. Cogeneration can be combined with other techniques, such as reducing the atmospheric pressure in the distillery. Because water boils at a lower temperature at lower atmospheric pressure, less energy is needed to evaporate it. Still, because distillation processes are so energy-expensive, they are used principally in energy-rich, water-scarce countries of the Middle East and North Africa. These countries are responsible for more than half the annual production of desalinated water.
reverse osmosis A desalination process that uses selectively permeable membranes and pressure to separate salts and water.
Reverse osmosis is a more energy-efficient desalination process that uses semipermeable membranes to separate salts and water. The membranes are permeable to water, allowing water molecules to pass across, but they are not permeable to salts. As in distillation, reverse osmosis creates two streams of water: (1) freshwater, which can be up to 99.7% pure water that is piped into the water distribution system, and (2) higher-salinity water that is discharged back into the environment. The energy needed to drive the process is used mainly for pressurizing the water fed into the system (Figure 6.32). As filters have improved, the pressure needed to run these systems has declined substantially, thereby reducing energy costs. However, there are also environmental impacts from the disposal of the salt brine, which has about twice the salinity of seawater, and the potential to harm marine life that gets sucked into the intakes.
OSMOSIS MOVES WATER ACROSS A SELECTIVELY PERMEABLE MEMBRANE
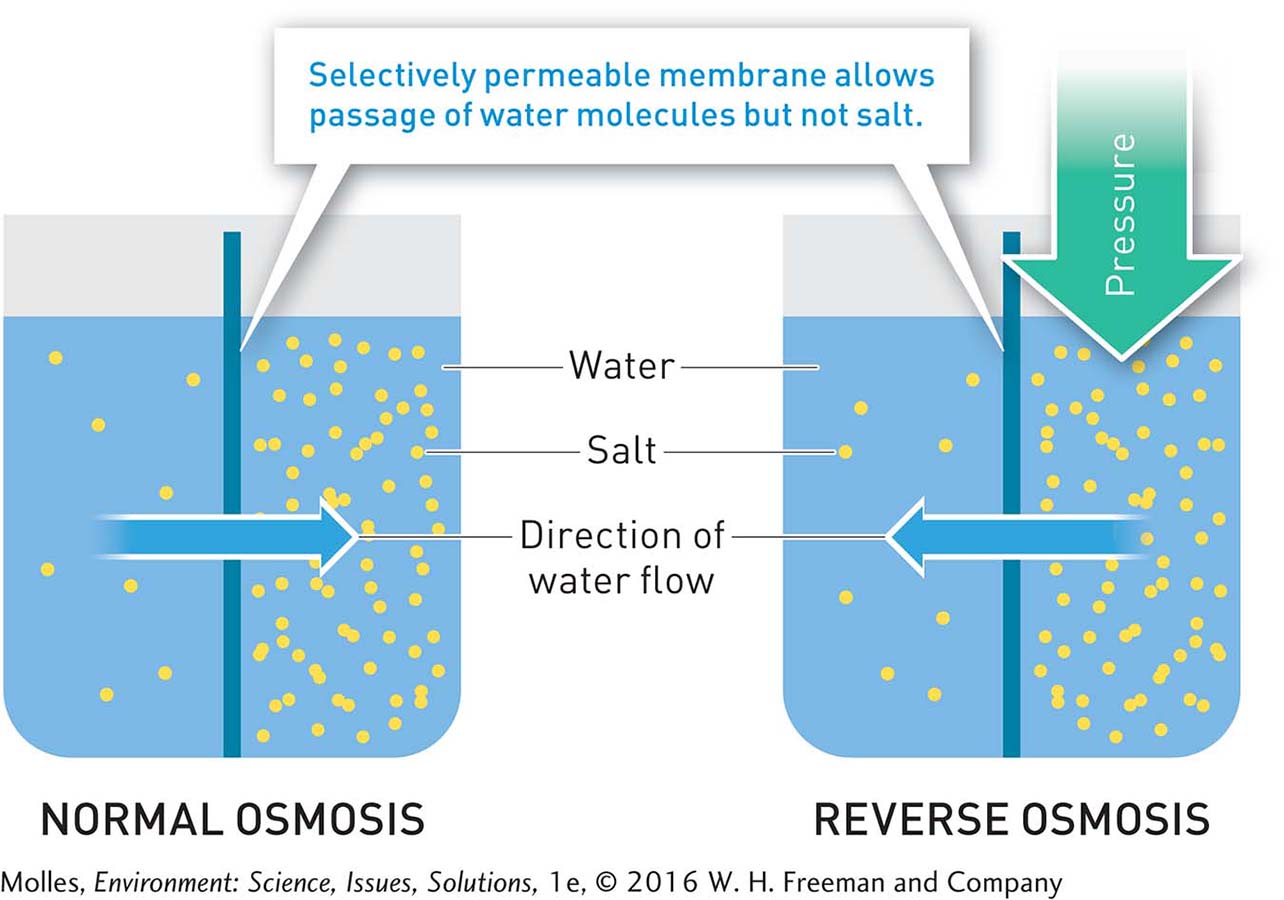
FIGURE 6.32 During the natural process of osmosis, water moves from the side of a selectively permeable membrane on which the concentration of salts is lower to the side on which the concentration of salts is higher. Reverse osmosis changes (“reverses”) this normal direction of flow by applying physical pressure to the side of the membrane with higher salt content, forcing water to move toward the side of the membrane with the lower salt content.
The Tampa Bay Desalination Plant
Tampa, Florida, has turned to desalination to supplement its traditional water supplies, opening the largest reverse osmosis plant in the United States in 2007 (Figure 6.33). Now fully operational, the plant has the capacity to produce 95 million liters (25 million gallons) of freshwater per day, approximately 10% of freshwater consumption for the Tampa Bay region.
THE TAMPA BAY SEAWATER DESALINATION PLANT USES REVERSE OSMOSIS

FIGURE 6.33 The metropolitan area of Tampa Bay, Florida, uses desalination to supply up to 10% of its needs for freshwater. The other 90% of freshwater for the Tampa Bay area comes from groundwater and treated river water.
(Courtesy Tampa Bay Water)
Some unique local and regional factors reduce the financial costs of the Tampa Bay desalination plant. First, because the salt content of the water in Tampa Bay averages approximately 25% less than full-strength seawater, the energy cost is reduced. Second, the plant is located next to an electrical generation plant that supplies low-cost power. The power plant also shares a variety of infrastructure and services with the desalination facility. For instance, the salt brine produced by the plant is diluted in the cooling water released by the power plant at a 70 :1 ratio. As a result, no increases in salinity have been observed in Tampa Bay, even when the desalination plant is operating at full capacity.

Why might Singapore elect to invest in a desalination plant even in the face of the prospect of purchasing water from neighboring Malaysia at a lower cost?
The costs of reverse osmosis have fallen sufficiently, as a result of more efficient filters and other refinements, to make it an attractive option to distillation plants. Facilities have been built by the island nations of Cyprus, Singapore, and Trinidad, where freshwater supplies are limited but seawater is abundant. In 2005 Singapore opened its Tuas reverse osmosis seawater desalination plant; its output capacity is nearly 50% higher than the Tampa Bay plant. Tuas can meet 10% of Singapore’s total water demand. Singapore plans to increase its desalination capacity 10-fold in order to meet 30% of its projected water needs by 2060. Even more impressive, desalination in Singapore has become so energy-efficient that its plants have been producing the world’s least costly desalinated water.
Even as costs for desalinating drinking water continue to decline, desalination will never be able to provide a solution for the great demands of agriculture for water. For instance, the U.S. Department of Agriculture estimates that irrigation is responsible for over 90% of consumptive water use in the western United States. We will discuss agricultural water use in Chapter 7.
Think About It
What are the major economic and environmental costs of a desalination plant?
Where would a desalination plant be most appropriate for supplementing a domestic water supply? Where would such a plant be least appropriate?




