9.3 Nuclear energy is released by atomic fission and fusion
nuclear energy A form of energy released when the nucleus of an atom breaks apart (nuclear fission), or when the nuclei of two atoms fuse (nuclear fusion).
nuclear fission A process in which the bonds holding the protons and neutrons that make up the nucleus of an atom are broken, resulting in the release of a large quantity of energy.
nuclear fusion A process in which the nuclei of two atoms fuse to form a new type of atom, releasing large amounts of energy.
Soon after the development of nuclear weapons in the middle of the 20th century, we found ways to transform this new, potent source of energy into electricity. Nuclear energy is released when the bonds holding the protons and neutrons making up the nucleus of an atom break, a process called nuclear fission (Figure 9.15). Nuclear energy is also released when the nuclei of two atoms fuse together under high temperature and pressure, a process called nuclear fusion. Nuclear fusion provides the fuel for stars, including our own Sun, and is the basis for one type of atomic weapon.
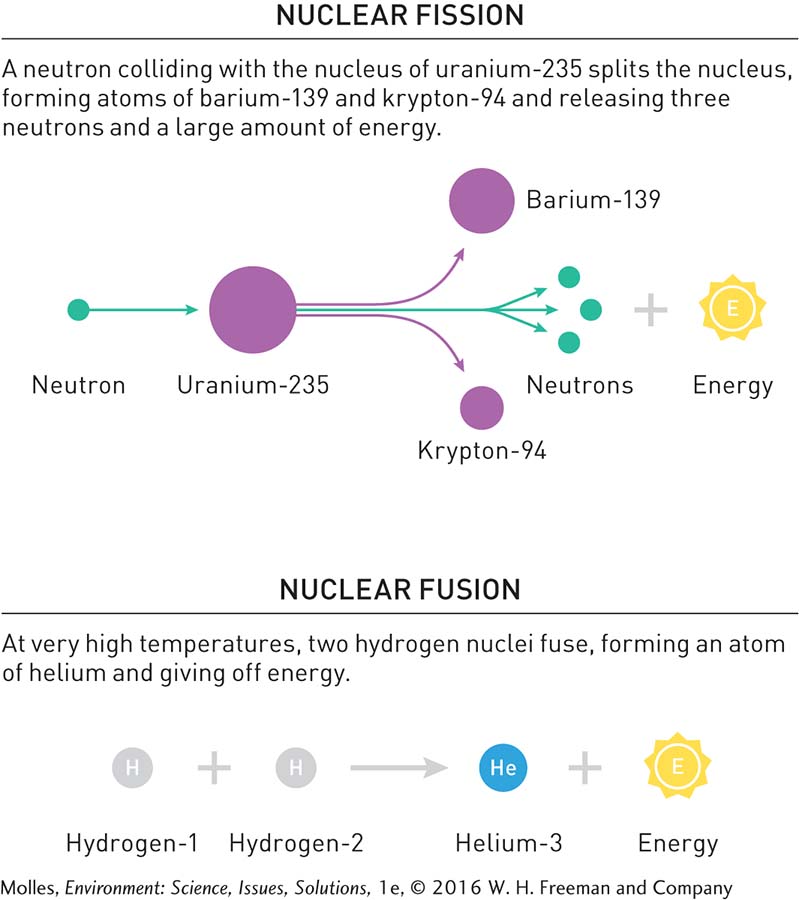
Nuclear bonds hold much more energy than chemical bonds. Therefore, the amount of energy released during nuclear fission is much greater than that released during the combustion of fossil fuels. For example, the energy content of a gram of uranium-
enrichment A nuclear process in which uranium-
Uranium, the fuel used in today’s nuclear power plants, is a nonrenewable resource. Uranium ore is mined from the ground and processed to produce a powdery, concentrated form known as yellowcake. The isotope used for fission, uranium-
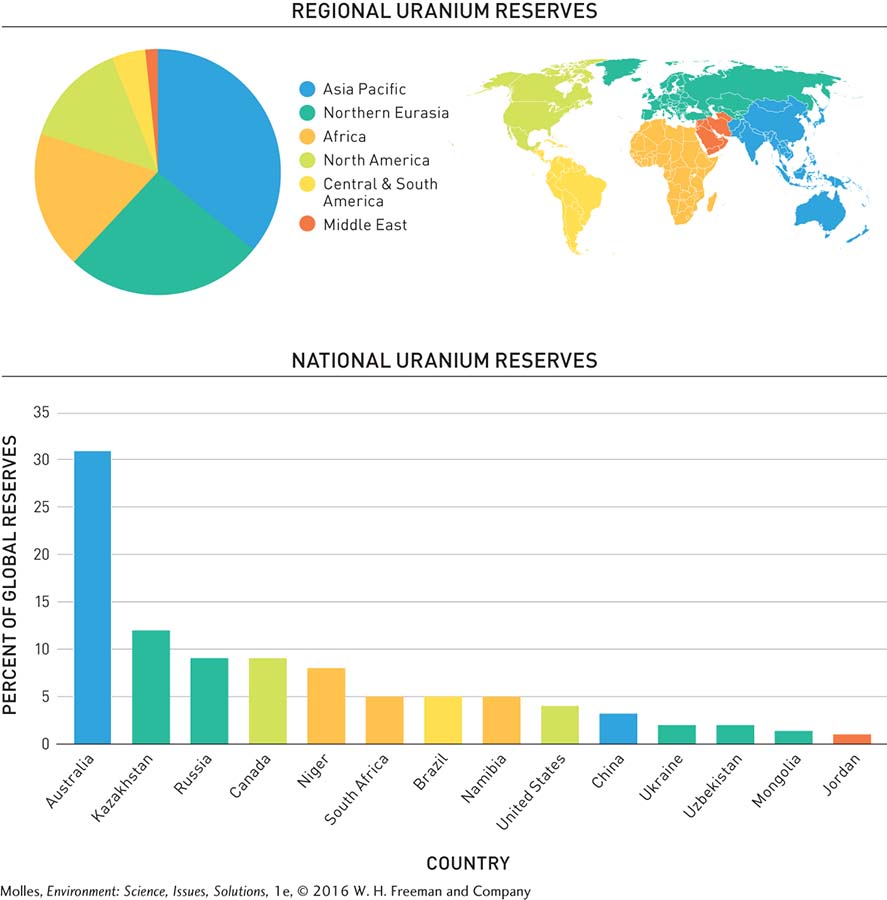
Approximately 97% of the known reserves of uranium have been found in just 15 countries (Figure 9.16). The largest known reserves, nearly one-
In contrast to nuclear fission, fusion doesn’t require uranium or other rare, radioactive materials, but rather a seemingly limitless fuel: hydrogen, which can be extracted from water (Figure 9.15). Presently, there are no commercial fusion reactors for power generation, but we will discuss its potential in the Solutions section of this chapter.
Nuclear Power Generation
In nuclear power plants, the heat energy released during the fission of uranium can be harnessed to generate electricity. This heat produces steam that drives a turbine connected to an electrical generator (Figure 9.17), similar to what we saw in the coal-
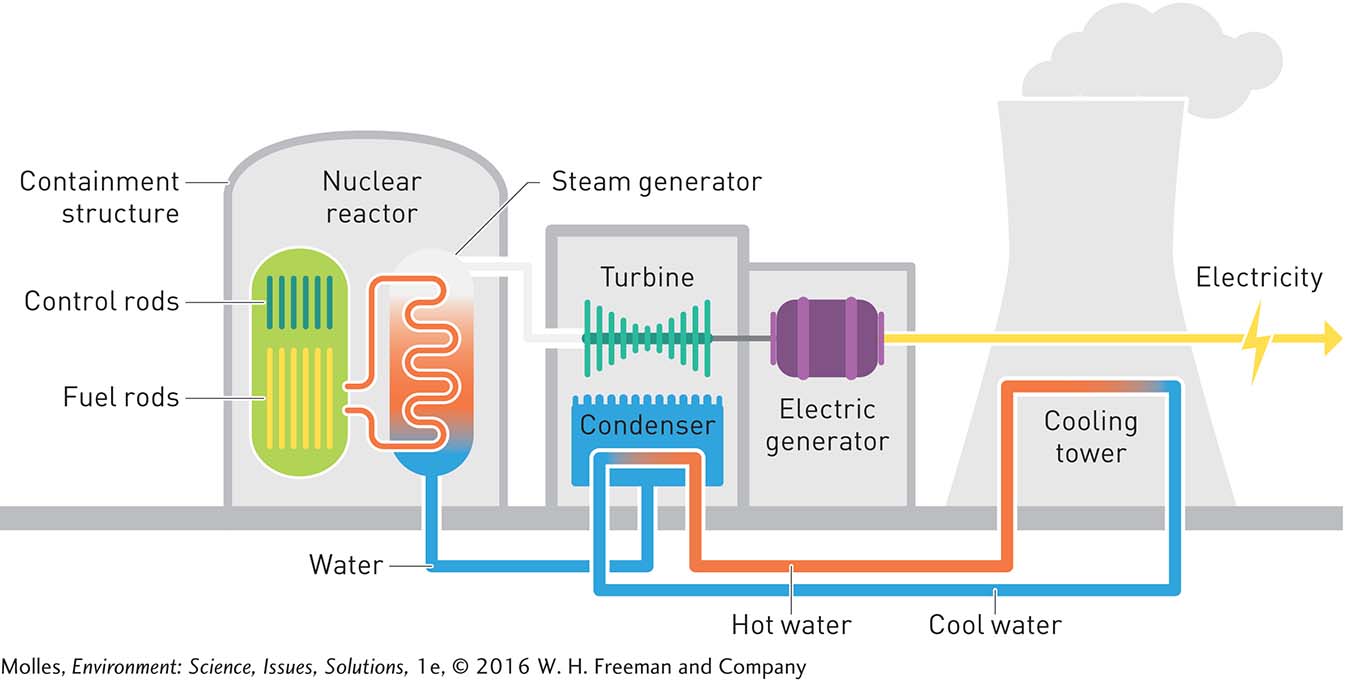
fuel rods Tubes containing small pellets of uranium-
moderator A substance (most commonly pressurized water) used in a nuclear reactor to reduce the speed at which neutrons travel.
Secure operation of a nuclear power station depends on controlling the speed of neutrons released during nuclear fission. Uranium-
273
control rods Long rods made of neutron-
The increasing number of fission reactions is an example of positive feedback (see Chapter 2, page 48). If uncontrolled, a chain reaction could generate so much heat that it would lead to a meltdown of a nuclear reactor. The chain reaction in nuclear reactors, and thus the amount of heat generated, must therefore be regulated using control rods, which are made of substances that absorb neutrons. Because they absorb neutrons, control rods can be inserted into a reactor core to slow the rate of nuclear fission or to shut down a reactor completely, either in an emergency or for repairs.
274
containment structure A steel and concrete enclosure designed to prevent the release of radioactive material in the case of a serious nuclear reactor accident.
The pressurized water circulating through the reactor is heated by the energy released during fission reactions. This heat produces the steam, which spins the turbine attached to a generator. Cooling water circulated through the turbine chamber cools the steam, condensing it back to liquid water. This water is returned to the steam generator, where it is again converted to steam. Notice that the three streams of water in the nuclear power plant—

Nuclear power is more expensive than power generated using coal or natural gas as energy sources. Why, then, have so many countries around the world invested heavily in the development of nuclear power plants?
Think About It
How are the processes that yield energy during chemical reaction different from the processes that yield nuclear energy?
What are some implications of uranium-
235 having approximately 3 million times the energy content compared to coal? How does increasing or decreasing the number of control rods inserted in a nuclear reactor influence the amount of heat generated in a nuclear reactor?
9.1–9.3 Science: Summary
The most commonly used sources of nonrenewable energy are fossil fuels in the form of coal, oil, and natural gas, which were formed over millions of years. Any source of heat sufficient to convert liquid water to steam can be used to generate electricity. Currently, the most common source of this heat is the combustion of fossil fuels, especially coal and natural gas. Oil can also be used to power internal combustion engines, which are found in vehicles and portable power generators. In the mid-