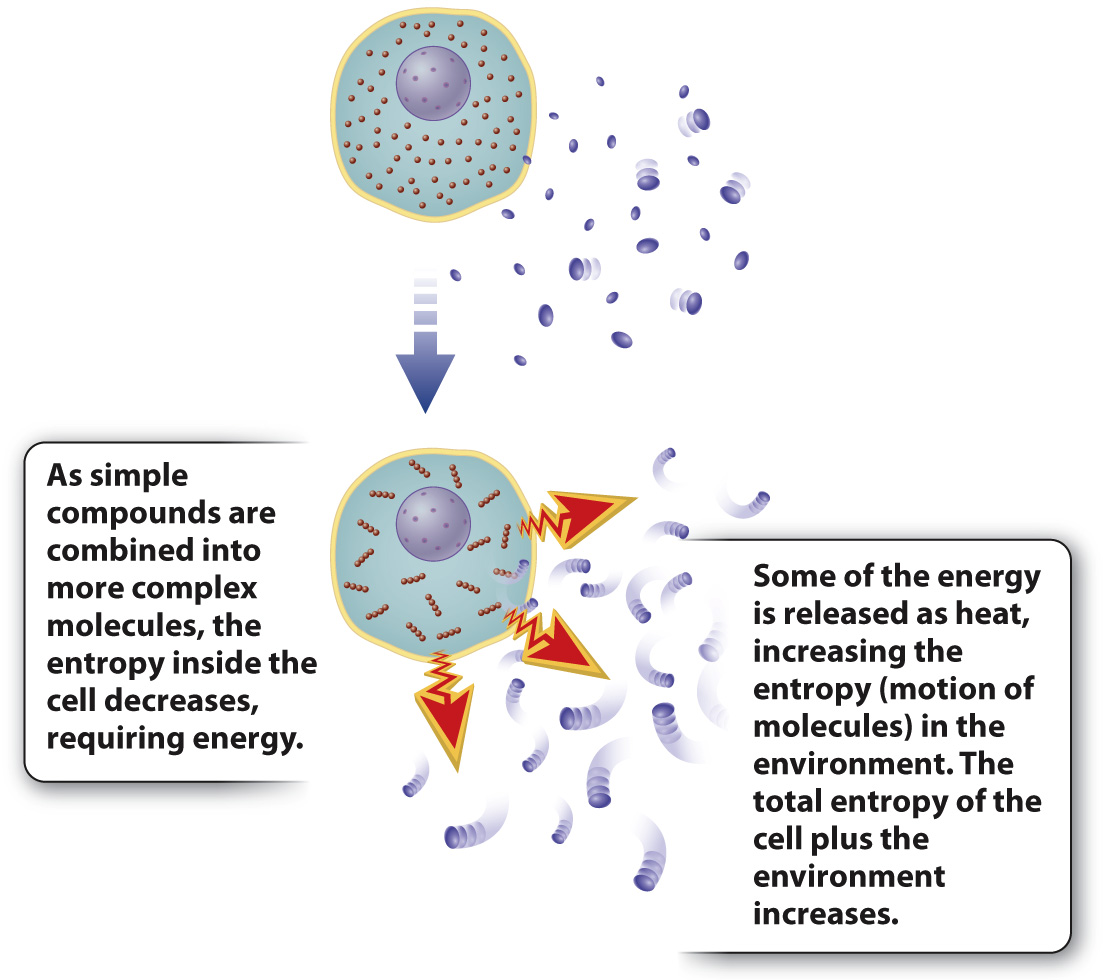
FIG. 1.7 Energy transformation and the second law of thermodynamics. The second law states that disorder in any system tends to increase. Entropy can decrease locally (inside a cell, for example) because the heat released increases disorder in the environment.