HOW DO WE KNOW?
FIG. 19.14
How does lactose lead to the production of active β-galactosidase enzyme?
BACKGROUND Active β-galactosidase enzyme is observed only in E. coli cells that are growing in the presence of lactose.
HYPOTHESIS One hypothesis is that the enzyme is always being produced, but is produced in an unstable form that breaks down rapidly in the absence of lactose. A second hypothesis is that the enzyme is stable, but is produced only in the presence of lactose.
EXPERIMENT François Jacob and Jacques Monod exposed a culture of growing cells to lactose and later removed it. They measured the amount of β-galactosidase present in the culture during the experiment.
RESULTS Almost immediately upon addition of lactose, β-galactosidase began to accumulate, and its amount steadily increased. When lactose was removed, the enzyme did not disappear immediately (as would be the case if it were unstable). Instead, the amount of enzyme remained the same as when lactose was present. This result is expected only if β-galactosidase is a stable enzyme that is synthesized when lactose is added and stops being synthesized when lactose is removed.
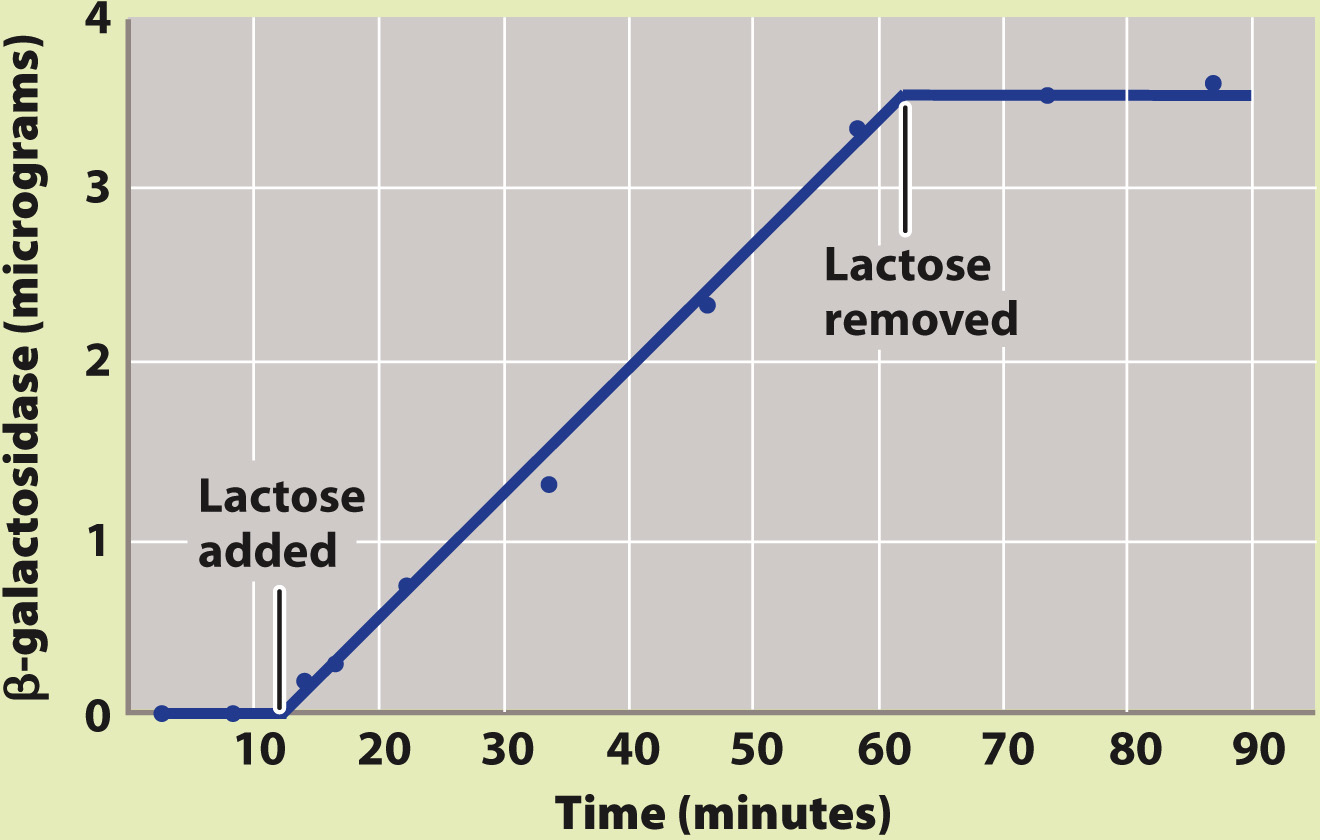
CONCLUSION Synthesis of β-galactosidase is turned on when lactose is added and turned off when lactose is removed, in support of the second hypothesis.
FOLLOW-
SOURCE Monod, J. 1965. “From Enzymatic Adaption to Allosteric Transitions.” Nobel Prize lecture. http:/