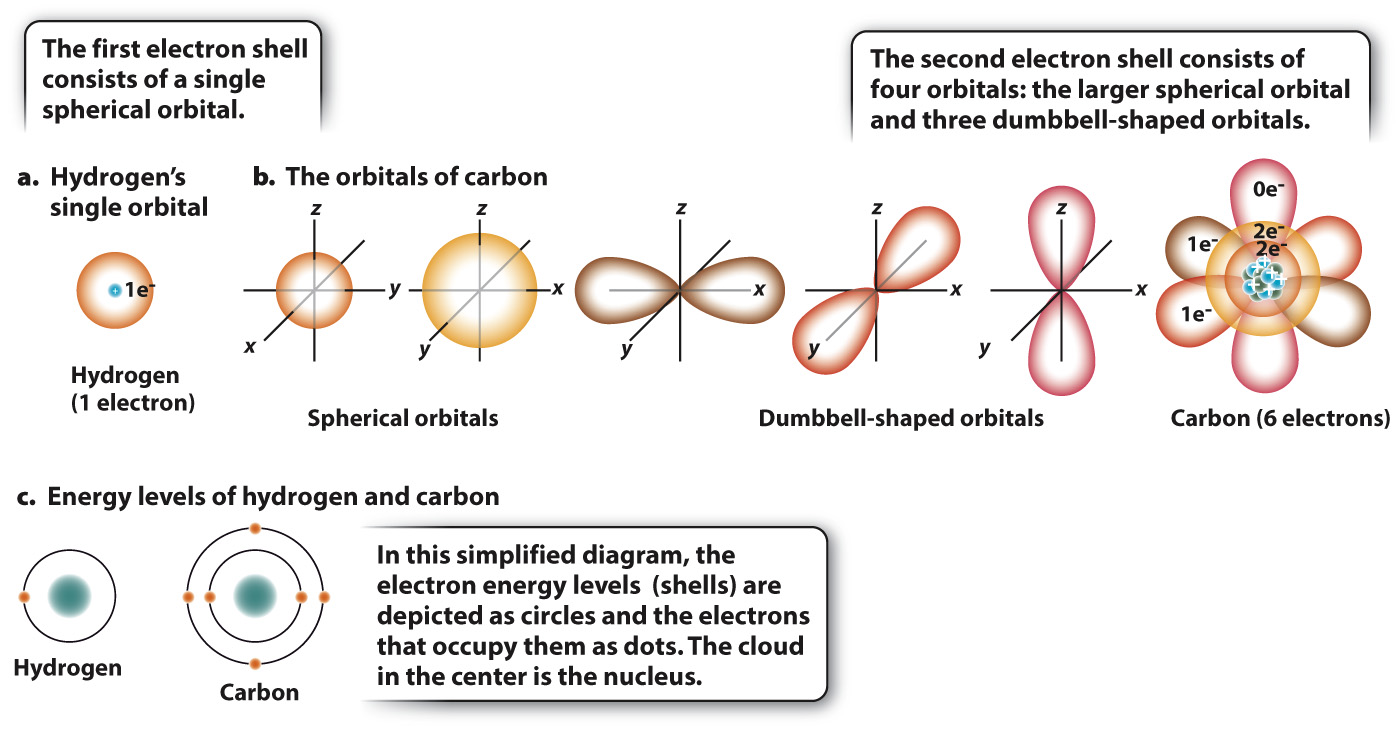
FIG. 2.2 Electron orbitals and energy levels (shells) for hydrogen and carbon. The orbital of an electron can be visualized as a cloud of points that is more dense where the electron is more likely to be. The hydrogen atom contains a single orbital, in a single energy level (a and c). The carbon atom has five orbitals, one in the first energy level and four in the second energy level (b and c).