HOW DO WE KNOW?
FIG. 39.12
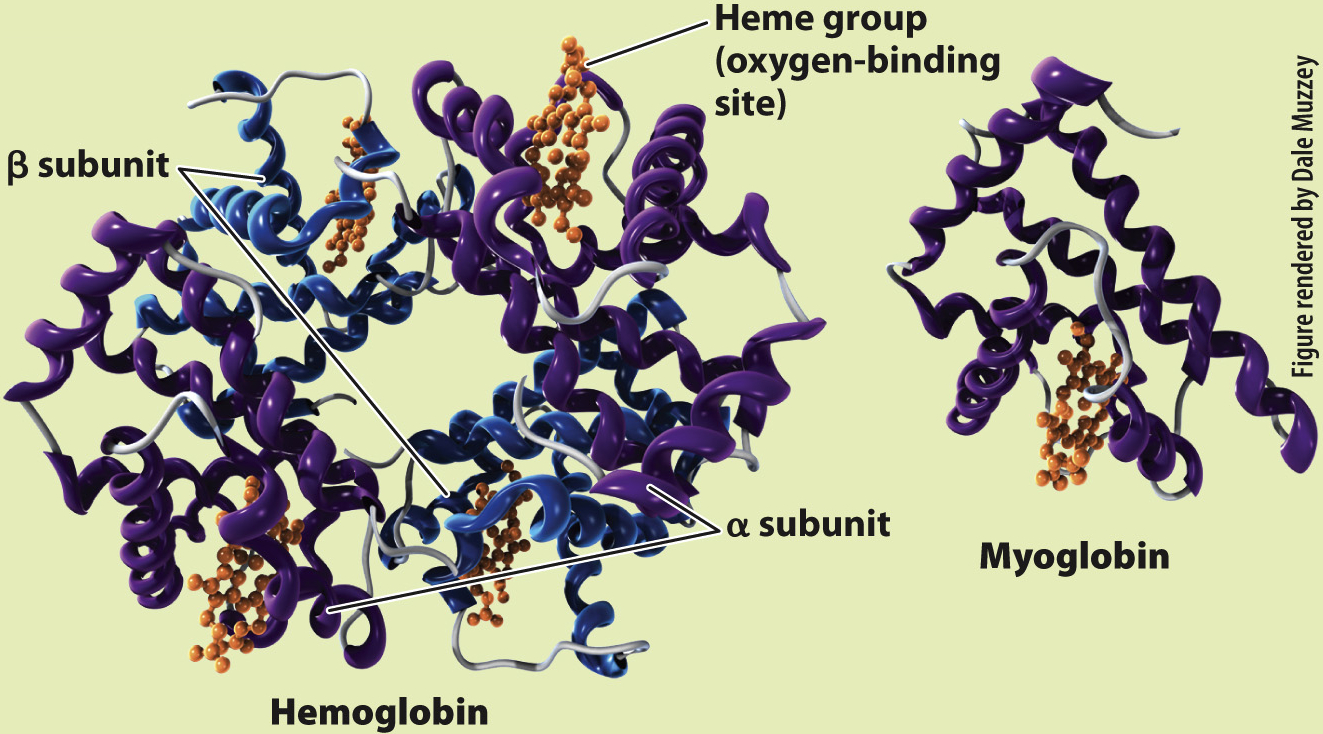
What is the molecular structure of hemoglobin and myoglobin?
BACKGROUND In the 1950s, the Austrian Max Perutz worked with John Kendrew in the Cavendish Laboratory of the University of Cambridge to determine the molecular structure of globular proteins. Perutz and Kendrew were interested in understanding how the structures of hemoglobin and myoglobin enabled binding and transport of O2.
EXPERIMENT Perutz and Kendrew developed and applied the new technique of X-
RESULTS Perutz and Kendrew showed that adult hemoglobin consists of four subunits, two α (alpha) and two β (beta) subunits. Each subunit contains a heme group that contains iron, which is the site of O2 binding. By contrast, myoglobin consists of only a single subunit with one heme group. These differences in molecular structure underlie the O2-binding and dissociation properties of the two O2 transport proteins.
FOLLOW-
SOURCES Kendrew J. C., and M. F. Perutz. 1948. “A Comparative X-