HOW DO WE KNOW?
FIG. 43.13
How is antibody diversity generated?
BACKGROUND Humans produce an estimated 10 billion different antibodies. Antibodies are proteins, encoded by genes. However, there are only about 25,000 genes in the human genome. How then can humans (and other vertebrates) produce such a diversity of antibodies from a limited set of genes? This was one of the central problems in immunology until the 1960s and 1970s.
HYPOTHESIS In 1965, American biologists William Dreyer and J. Claude Bennett proposed a model in which there are many copies of two gene segments, which they called the V (variable) and C (constant) segments. An antibody gene is assembled by recombining a single copy of each of these segments. At the time, there was no direct experimental evidence to support this hypothesis.
EXPERIMENT In 1976, Japanese immunologists Nobumichi Hozumi and Susumu Tonegawa tested the Dreyer and Bennett hypothesis. Using mice as a model system, they isolated DNA from embryonic cells (before recombination was thought to occur) and from adult B-
RESULTS Hozumi and Tonegawa found that the V and C regions were far apart in the DNA of embryonic cells and close together in adult cells, suggesting that the gene segments were brought together during B cell differentiation.
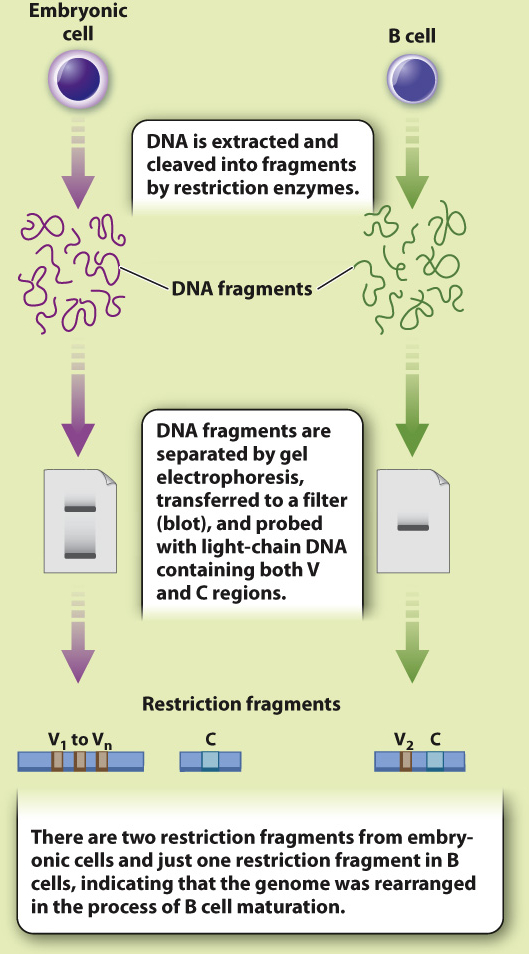
CONCLUSION Antibody genes are assembled by recombination of individual gene segments, providing a mechanism for generating antibody diversity.
FOLLOW-
SOURCES Dreyer, W. J., and J. C. Bennett. 1965. “The Molecular Basis of Antibody Formation: A Paradox.” Proceedings of the National Academy of Sciences 54:864–