HOW DO WE KNOW?
FIG. 6.16
Do enzymes form complexes with substrates?
BACKGROUND The idea that enzymes form complexes with substrates to catalyze a chemical reaction was first proposed in 1888 by the Swedish chemist Svante Arrhenius. One of the earliest experiments that supported this idea was performed by American chemist Kurt Stern in the 1930s. He studied an enzyme called catalase, which is very abundant in animal and plant tissues. In the conclusion of his paper, he wrote, “It remains to be seen to which extent the findings of this study apply to enzyme action in general.” Therefore, another experiment that analyzes a different enzyme and technique are described here.
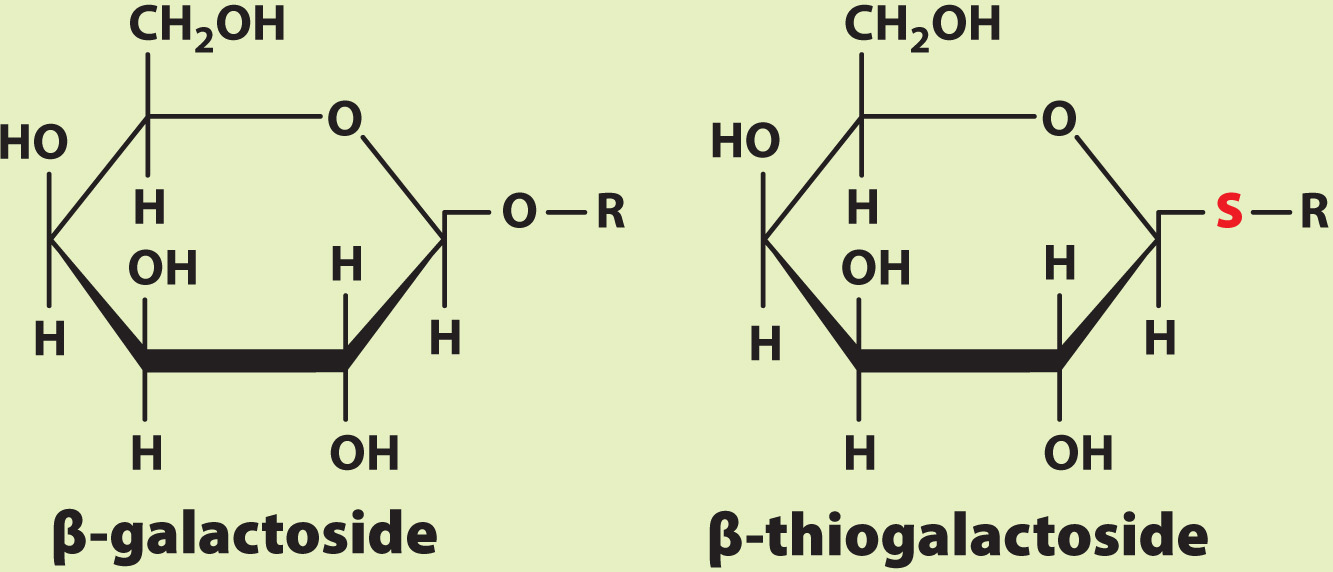
The enzyme β-galactosidase catalyzes the cleavage of the glycosidic bond that links galactose to glucose in the disaccharide lactose. Lactose belongs to a family of molecules called β-galactosides since it contains a galactose unit attached to the rest of the molecule by a glycosidic bond. In a related compound called β-thiogalactoside, the oxygen atom in the glycosidic bond is replaced by sulfur. The enzyme β-galactosidase binds β-thiogalactoside but cannot cleave or release it.
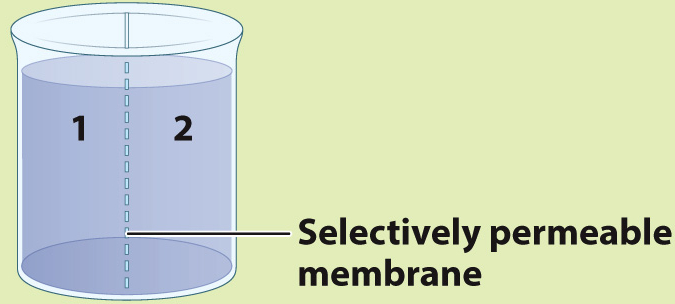
METHOD A container is separated into two compartments by a selectively permeable membrane. The membrane is permeable to β-galactoside and β-thiogalactoside, but not permeable to the enzyme.
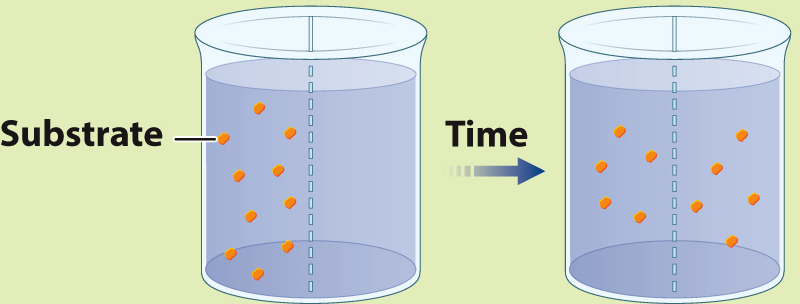
EXPERIMENT 1 Radioactively labeled β-thiogalactoside (S) is added to compartment 1 and the movement of S is followed by measuring the level of radioactivity in the two compartments.
RESULT Over time, the level of radioactivity is the same in the two compartments.
EXPERIMENT 2 Radioactively labeled β-thiogalactoside (S) is added to compartment 1, enzyme (E) is added to compartment 2, and the movement of S is followed by measuring the level of radioactivity in the two compartments.
RESULT Over time, the level of radioactivity is greater in compartment 2 than in compartment 1.
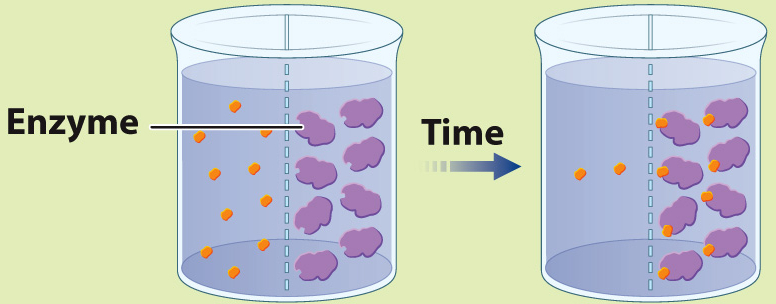
CONCLUSION These results can be explained if the substrate diffuses from compartment 1 to compartment 2, forms a complex with the enzyme, and is not released because the enzyme cannot catalyze the conversion of substrate to product. In other words, E and S form a complex.
SOURCES Adapted from Doherty, D. G., and F. Vaslow. 1952. “Thermodynamic Study of an Enzyme–