Telomerase restores tips of linear chromosomes shortened during DNA replication.
A circular DNA molecule can be replicated completely because it has no ends, and the replication forks can move completely around the circle (Fig. 12.10). Linear DNA molecules have ends, however, and at each round of DNA replication the ends become slightly shorter. The reason for the shortening is illustrated in Fig. 12.11.
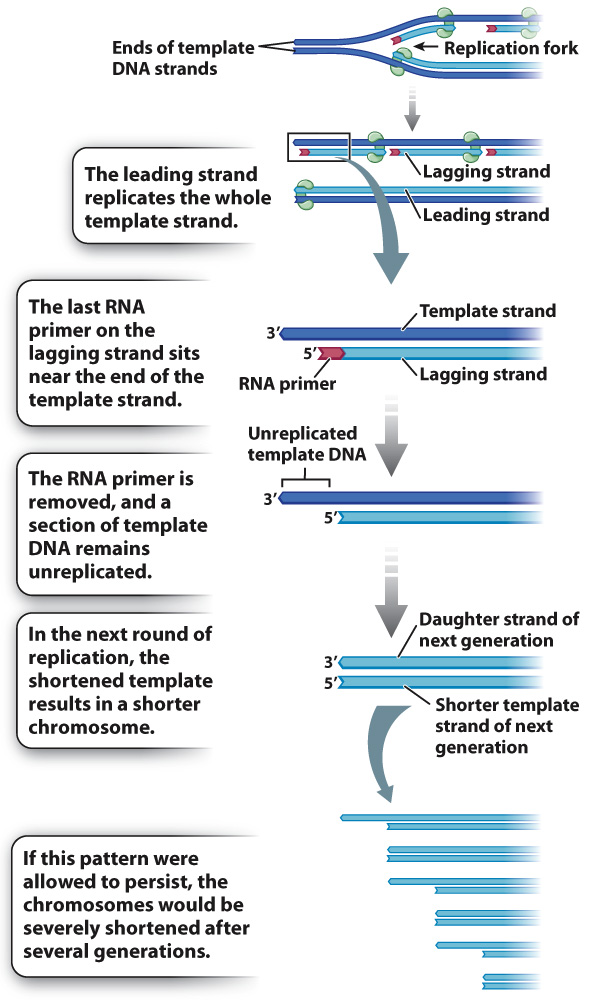
Recall that each fragment of newly synthesized DNA starts with an RNA primer. On the leading strand, the only primer required is at the origin of replication when synthesis begins. The leading strand is elongated in the same direction as the moving replication fork and is able to replicate the template strand all the way to the end. But on the lagging strand, which grows away from the replication fork, many primers are required, and the final RNA primer is synthesized about 100 nucleotides from the 3′ end of the template. Because this primer initiates synthesis of the final Okazaki fragment of the lagging strand, there is no other Okazaki fragment to synthesize the missing 100 base pairs and remove the primer. Therefore, when DNA replication is complete, the new daughter DNA strand (light blue in Fig. 12.11) will be missing about 100 base pairs from the tip (plus a few more owing to the length of the primer). When this daughter strand is itself replicated, the newly synthesized strand must terminate at the shortened end of the template strand, and so the new duplex molecule is shortened by about 100 base pairs from the original parental molecule. The strand shortening in each round of DNA replication is a problem because without some mechanism to restore the tips, the DNA in the chromosome would eventually be nibbled away to nothing.
256
Fig. 12.12 illustrates the mechanism that eukaryotic organisms have evolved to solve the problem of shortened ends. Each end of a eukaryotic chromosome is capped by a repeating sequence called the telomere. The repeating sequence that constitutes the telomere differs from one group of organisms to the next, but in the chromosomes of humans and other vertebrate it consists of the sequence 3′-GGGATT-
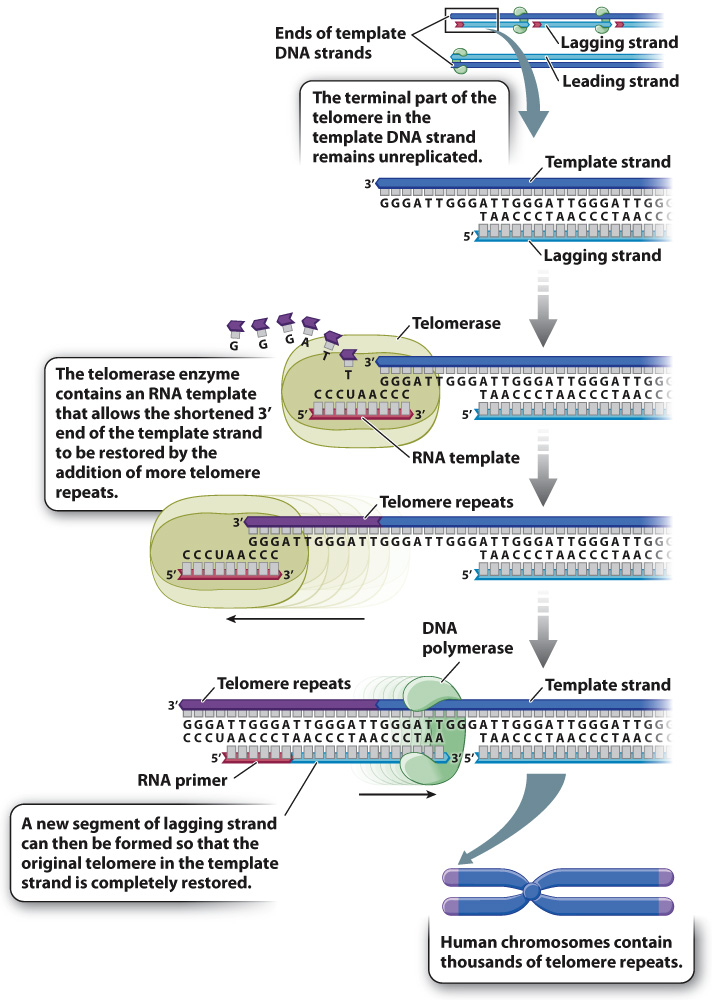
As shown in Fig. 12.12, the telomerase replaces the lost telomere repeats using its RNA molecule as a guide to add successive DNA nucleotides to the 3′ end of the template strand. Once the 3′ end of the template strand has been elongated, an RNA primer and the complementary DNA strand are synthesized. In this way the original telomere is completely restored. Because there are no genes in the telomere, the slight shortening and subsequent restoration that take place have no harmful consequences.
Telomerase activity differs from one cell type to the next. It is fully active in germ cells, which produce sperm or eggs, and also in stem cells, which are undifferentiated cells that can undergo an unlimited number of mitotic divisions and can differentiate into any of a large number of specialized cell types. Stem cells are found in embryos, where they differentiate into all the various cell types. Stem cells are also found in some tissues of the body after embryonic development, where they replenish cells that have a high rate of turnover, such as blood and intestinal cells, and play a role in tissue repair.
In contrast to the high activity of telomerase in germ cells and stem cells, telomerase is almost inactive in most cells in the adult body. In these cells, the telomeres are actually shortened by about 100 base pairs in each mitotic division. Telomere shortening limits the number of mitotic divisions that the cells can undergo because human cells stop dividing when their chromosomes have telomeres with fewer than about 100 copies of the telomere repeat. Adult somatic cells can therefore undergo only about 50 mitotic divisions until the telomeres are so short that the cells stop dividing.
Many biologists believe that the limit on the number of cell divisions explains in part why our tissues become less youthful and wounds heal more slowly with age. The telomere hypothesis of aging is still controversial, but increasing evidence suggests that it is one of several factors that lead to aging. The flip side of the coin is observed in cancer cells, in which telomerase is reactivated and helps support the uncontrolled growth and division of abnormal cells.
257