Some mutations are due to the insertion of a transposable element.
300
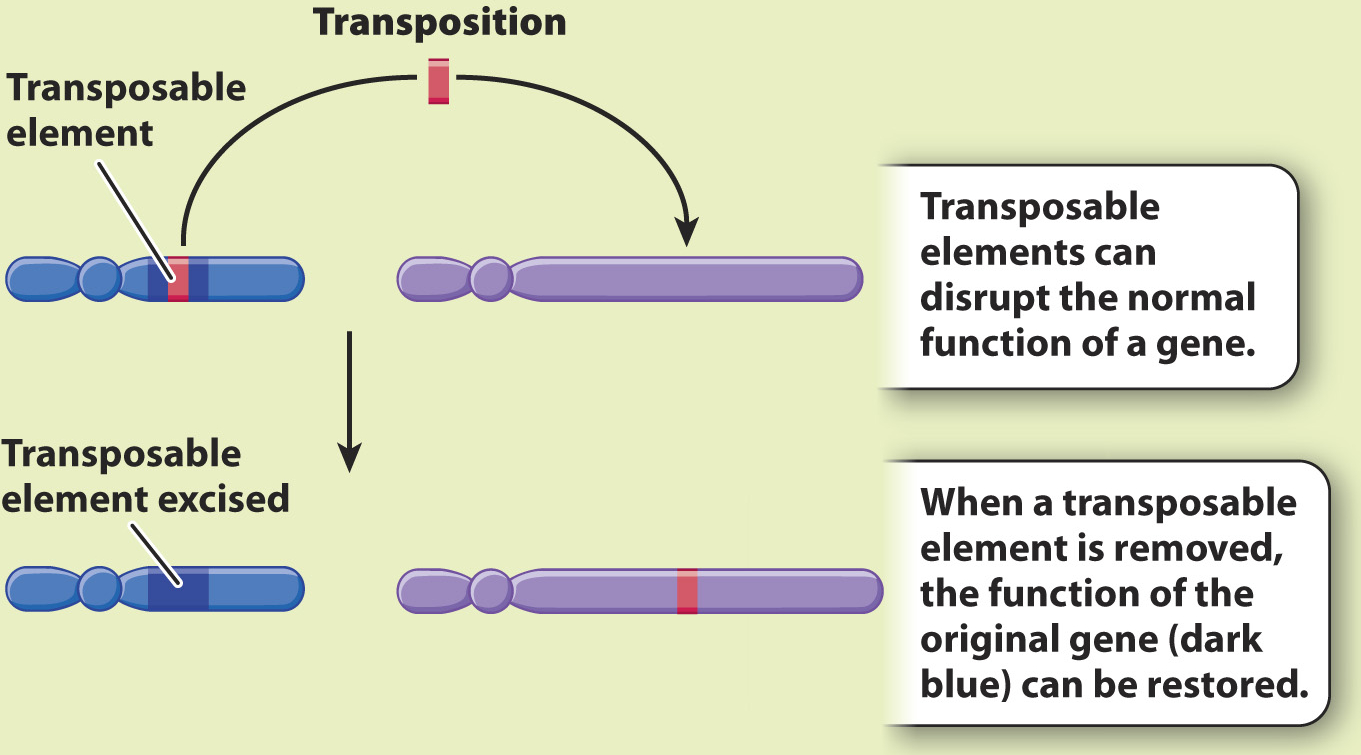
An important source of new mutations in many organisms is the insertion of movable DNA sequences into or near a gene. Such movable DNA sequences are called transposable elements or transposons. As we saw in Chapter 13, the genomes of virtually all organisms contain several types of transposable element, each present in multiple copies per genome.
Transposable elements were discovered by American geneticist Barbara McClintock in the 1940s (Fig. 14.11). She studied corn (maize) because genetic changes that affect pigment formation can be observed directly in the kernels. The normal color of maize kernels is purple. Each kernel consists of many cells, and if no mutations affecting pigmentation occur in a kernel, it will be uniformly purple. The kernel pigments are synthesized by several different enzymes in a metabolic pathway, and any of these enzymes can be rendered nonfunctional by mutations in their genes, including the insertion of transposable elements. Since McClintock’s work, we have learned that most transposable elements are segments of DNA a thousand or more base pairs long. When such a large piece of DNA inserts into a gene, it can interfere with transcription, cause errors in RNA processing, or disrupt the open reading frame. The result in the case of maize is that the cell is unable to produce pigment, and so the kernels will be yellow.
HOW DO WE KNOW?
FIG. 14.11
What causes sectoring in corn kernels?
BACKGROUND In the late 1940s, Barbara McClintock discovered what are now called transposable elements, DNA sequences that can move from one position to any other in the genome. She studied corn (Zea mays). Wild-

HYPOTHESIS McClintock hypothesized that the yellow mutant color resulted from a transposable element, which she called Dissociator (Ds), jumping into a site near or in the anthocyanin gene and disrupting its function. She attributed the purple streaks to cell lineages in which the transposable element had jumped out again, restoring the anthocyanin gene.
EXPERIMENT AND RESULTS By a series of genetic crosses, McClintock showed that the genetic instability of Ds was due to something on another chromosome that she called Activator (Ac). She set up crosses in which she could track the Ac-
CONCLUSION McClintock’s conclusion is illustrated in Fig. 14.11c: Transposable elements can be excised from their original position in the genome and inserted into another position.
FOLLOW-
SOURCE McClintock, B. 1950. “The Origin and Behavior of Mutable Loci in Maize.” Proceedings of the National Academy of Sciences of the USA. 36:344–
301
Among mutants affecting kernel pigmentation, McClintock observed certain mutants that resulted in kernels that were mostly yellow but speckled with purple. She suspected that these particular mutants might result from a transposable element jumping into and out of a gene. Each colored sector consists of a lineage of daughter cells from a single ancestral cell in which pigment synthesis had been restored. McClintock realized that, just as the inability of the kernel cells to produce pigment was caused by a transposable element jumping into a gene, restoration of that ability could be caused by the transposable element jumping out again. Her hypothesis that transposable elements are responsible for the pigment mutations was confirmed when she found that in cells where pigment had been restored, mutations affecting other genes had also occurred. She deduced that these other mutations were due to a transposable element jumping out of a pigment gene and into a different gene in the same cell.
The movement, or transposition, of transposable elements occurs by different mechanisms according to the type of transposon (Chapter 13). McClintock’s original discovery was of a DNA transposon that transposes by a cut-
Not all transposons undergo transposition by a cut-