The living and nonliving worlds follow the same chemical rules and obey the same physical laws.
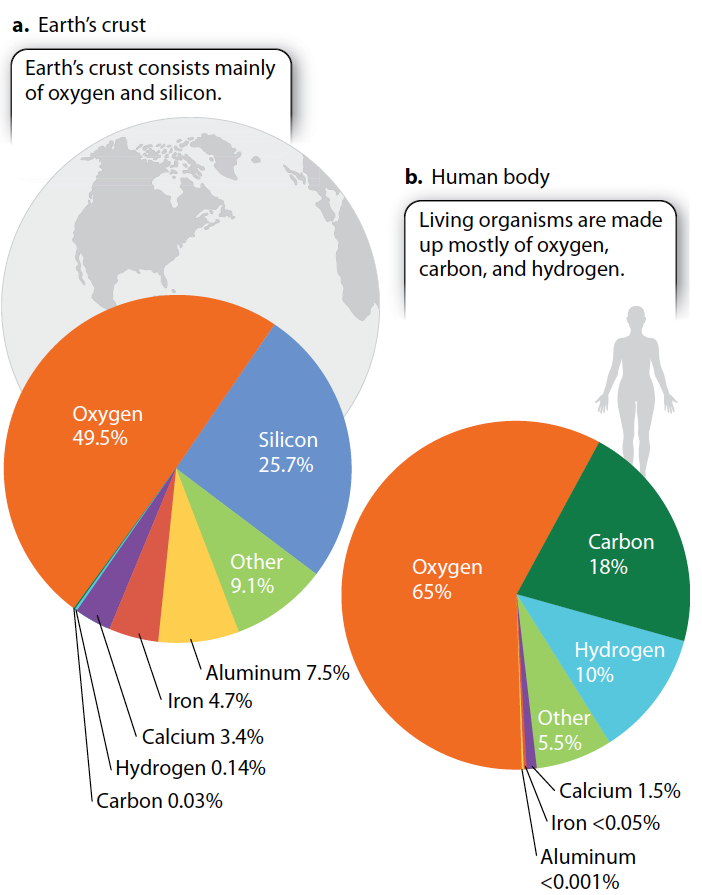
The chemical elements found in rocks and other nonliving things are no different from those found in living organisms. In other words, all the elements that make up living things can be found in the nonliving environment—
9
All living organisms are subject to the physical laws of the universe. Physics helps us to understand how animals move and why trees don’t fall over; it explains how redwoods conduct water upward through their trunks and how oxygen gets into the cells that line your lungs. Indeed, two laws of thermodynamics, both of which describe how energy is transformed in any system, determine how living organisms are able to do work and maintain their spatial organization.
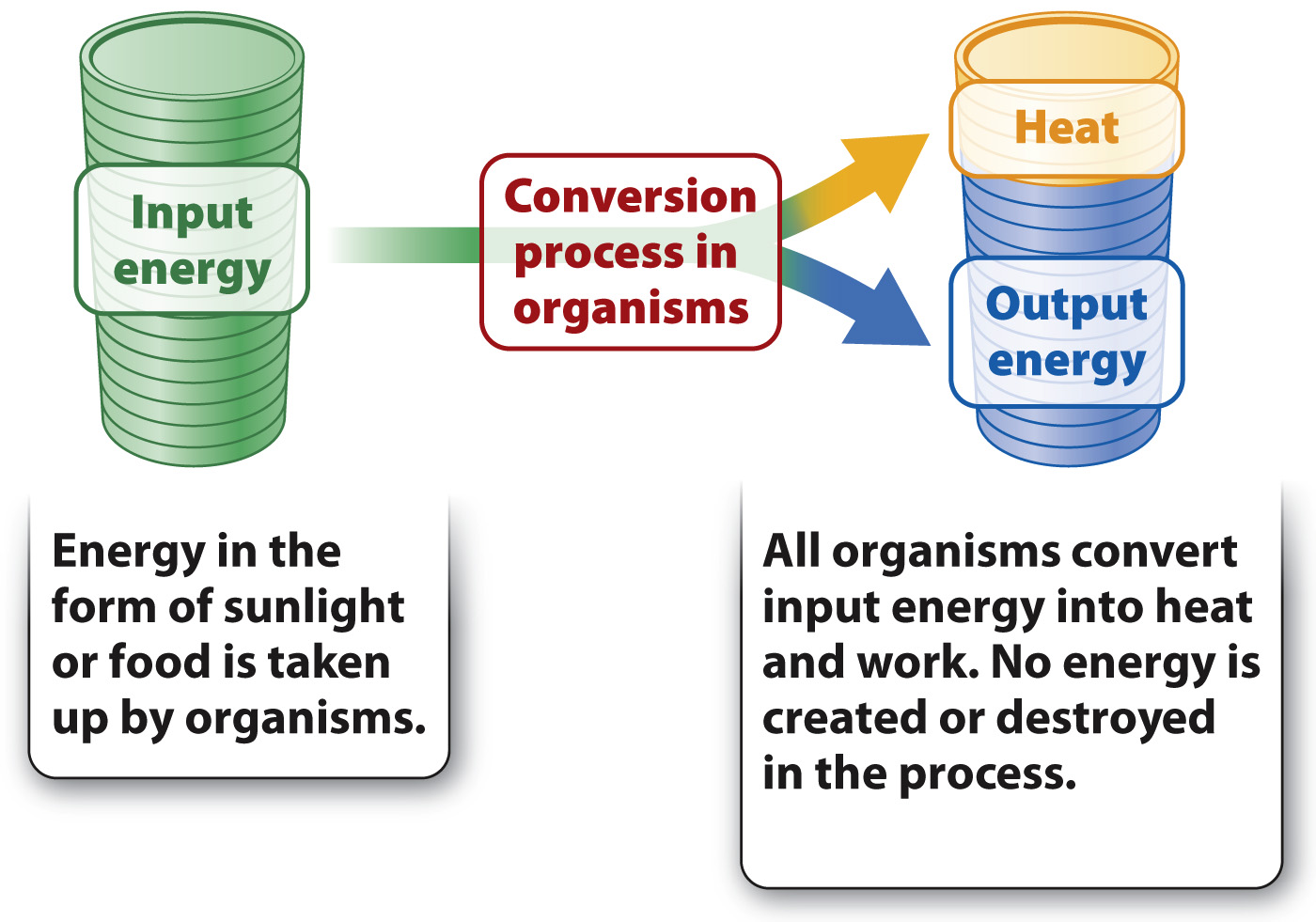
The first law of thermodynamics states that energy can neither be created nor destroyed; it can only be transformed from one form into another. In other words, the total energy in the universe is constant, but the form that energy takes can change. Living organisms are energy transformers. They acquire energy from the environment and transform it into a chemical form that cells can use. All organisms obtain energy from the sun or from chemical compounds. Some of this energy is used to do work—
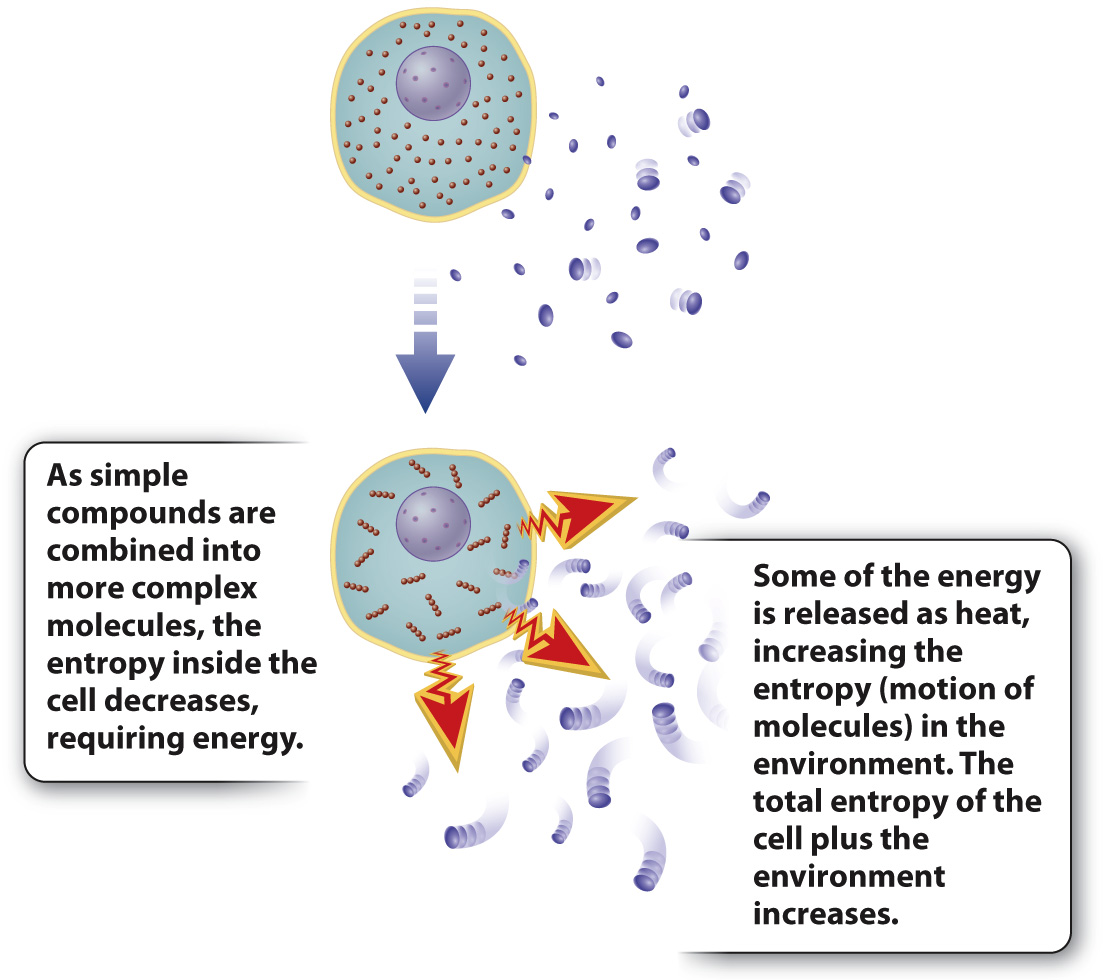
The second law of thermodynamics states that the degree of disorder (or the number of possible positions and motions of molecules) in the universe tends to increase. Think about a box full of marbles distributed more or less randomly; if you want to line up all the red ones or blue ones in a row, you have to do work—
Living organisms are highly organized. As with lining up marbles in a row, energy is needed to maintain this organization. Given the tendency toward greater disorder, the high level of organization of even a single cell would appear to violate the second law. But it does not. The key is that a cell is not an isolated system and therefore cannot be considered on its own; it exists in an environment. So we need to take into account the whole system, the cell plus the environment that surrounds it. As energy is harnessed by cells, only some is used to do work; the rest is dissipated as heat (Fig. 1.6). That is, conversion of energy from one form to another is never 100% efficient. Heat is a form of energy, so the total amount of energy is conserved, as dictated by the first law. In addition, heat corresponds to the motion of small molecules—
10