Case 1. The First Cell: Life’s Origins
25
CASE 1
Deep underground, in Mexico’s Cueva de Villa Luz, the cave walls drip with slime. The rocky surfaces are teeming with colonies of mucus-

Snottites might be stomach turning, but they’re intriguing, too. The organisms that produce snottites are called extremophiles because they live in places where humans and most other animals cannot survive. Such microorganisms may tell us something about life when Earth was young.
All cells require an archive of information, a membrane to separate the inside of the cell from its surroundings, and the ability to gather materials and harness energy from the environment.
From cave-
When and how life originated are some of the biggest questions in biology. Earth is nearly 4.6 billion years old. Chemical evidence from 3.5-
How did the first living cell arise? Scientists generally accept that life arose from nonliving materials—
All cells require an archive of information, a membrane to separate the inside of the cell from its surroundings, and the ability to gather materials and harness energy from the environment. In modern organisms, the cell’s information archive is DNA, the double-
DNA is critical, and it’s complex. Among the organisms alive today, the smallest known genome belongs to the bacterium Carsonella rudii. Even that genome contains nearly 160,000 DNA base pairs. How could such sophisticated molecular systems have arisen?
The likely answer to that question is: step by step. Laboratory experiments have shown how precursors to nucleic acids might have come together under chemical conditions present on the young Earth. It’s exceedingly unlikely that a molecule as complex as DNA was employed by the very first living cells. As you’ll see in the chapters that follow, scientists have gathered evidence suggesting that RNA, rather than DNA, stored information in early cells and, indeed, did much more than that, catalyzing chemical reactions much as proteins do today.
26
While some kind of information archive was necessary for life to unfold, there is more to the story. Living things must have a barrier that separates them from their environment. All cells, whether found as single-
Once again, scientists can only guess at how the first cell membranes came about, but research shows that the molecules that make up modern membranes possess properties that may have led them to form spontaneously on the early Earth. At first, the membranes were probably quite simple—
A third essential characteristic of living things is the ability to harness energy from the environment. Here, too, it’s feasible that a series of natural chemical processes led to entities that could achieve this feat. Simple reactions that produced molecular by-
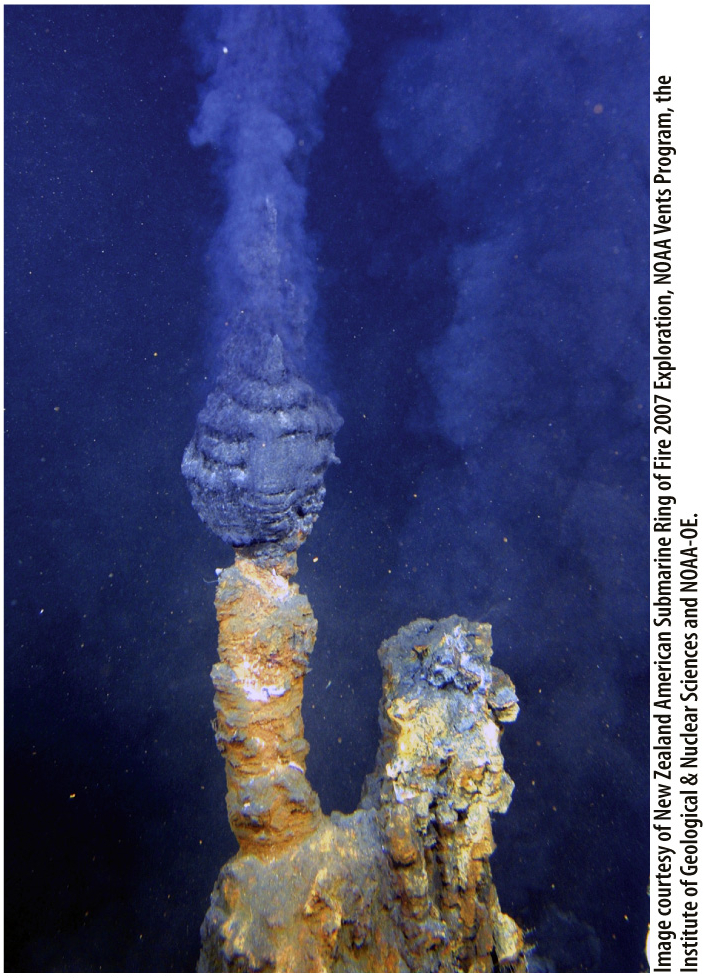
Such a series of events may sound unlikely. However, some scientists argue that given the chemicals present on the early Earth, it was likely—
That’s one reason researchers are so interested in studying organisms found today in extreme environments. The sulfur-
27
Did life arise just once? Or could it have started up and died out several times before it finally got a foothold? If, given Earth’s early chemistry, life here was inevitable, could it have arisen elsewhere in the universe? The study of life’s origins produces many more questions than answers—
CASE 1 QUESTIONS
Special sections in Chapters 2–
How did the molecules of life form? See page 45.
What was the first nucleic acid molecule, and how did it arise? See page 59.
How did the genetic code originate? See page 83.
How did the first cell membranes form? See page 91.
What naturally occurring elements might have spurred the first reactions that led to life? See page 128.
What were the earliest energy-
harnessing reactions? See page 139. How did early cells meet their energy requirements? See page 146.
How did early cells use sunlight to meet their energy requirements? See page 170.