Case 3. You, from A to T: Your Personal Genome
244
CASE 3

As an American Studies major at Georgetown University, Claudia Gilmore had plenty of experience taking exams. But at the age of 21, she faced an altogether different kind of test—
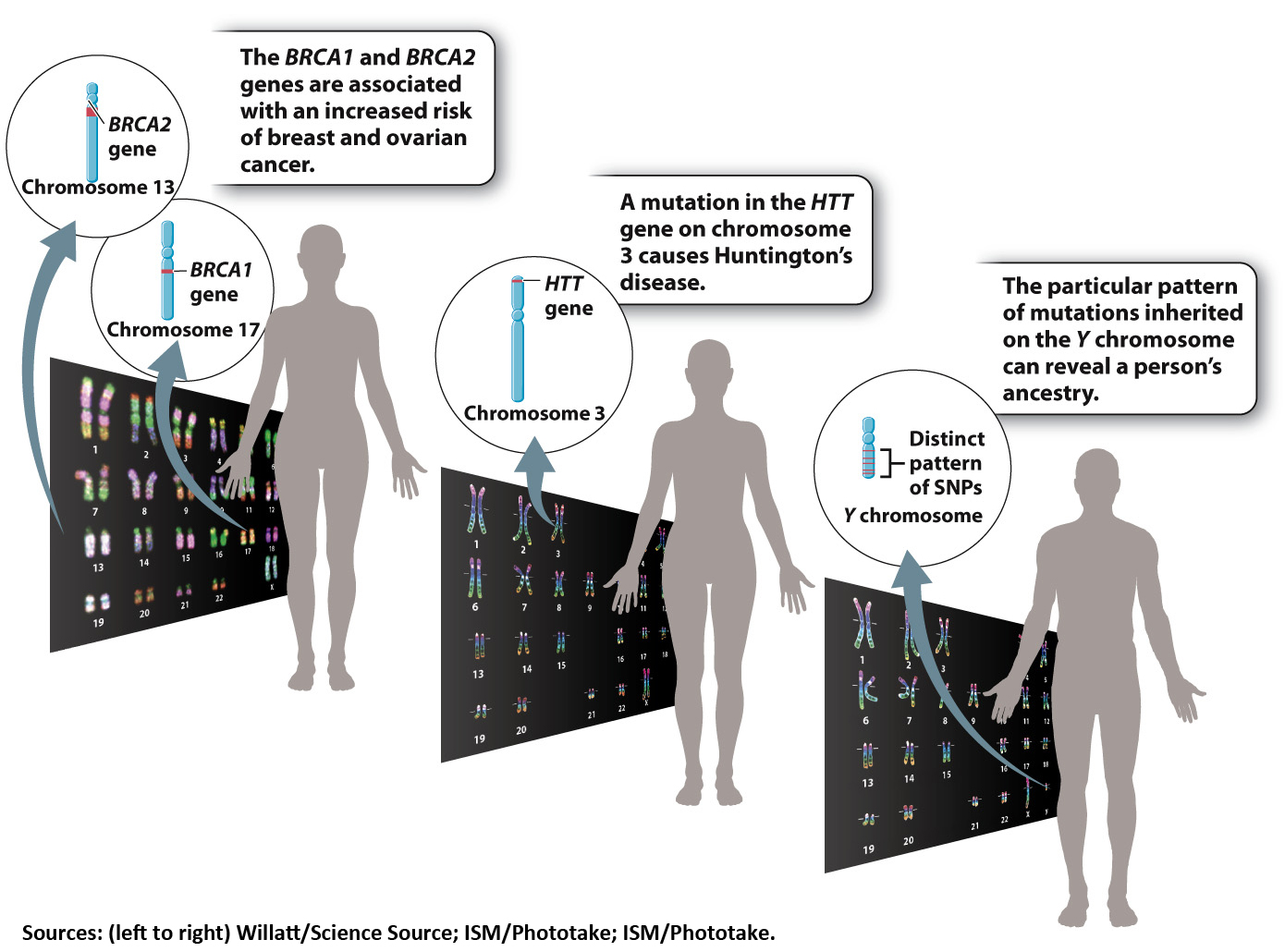
Gilmore had decided to be tested for a mutation in a gene known as BRCA1. Specific mutations in the BRCA1 and BRCA2 genes are associated with an increased risk of breast and ovarian cancers.
Gilmore’s grandmother had battled breast cancer and later died from ovarian cancer. Before her death, she had tested positive for the BRCA1 mutation. Her son, Gilmore’s father, was tested and discovered he had inherited the mutation. After talking with a genetic counselor to help her understand the implications of the test, Gilmore gave a sample of her own blood and crossed her fingers.
Mutations that increase the risk of developing a particular disease are called genetic risk factors.
“I had a fifty-
Unfortunately, it could. Two weeks after her blood was drawn, she learned that she, too, carried the mutated gene.
Genetic testing is becoming increasingly common—
In reality, there isn’t one single human genome. Everyone on Earth (with the exception of identical twins) has his or her own unique genetic sequence. Your personal genome, consisting of two sets of chromosomes, is the blueprint that codes for your hair color, the length of your nose, and your susceptibility to certain diseases. On average, any two human genomes are 99.9% identical, meaning that they differ at about 3 million base pairs.
Oftentimes, those differences have no impact on health. In some cases, however, a particular genetic signature is associated with disease. Sometimes a gene mutation makes a given illness inevitable. Certain mutations in a gene called HTT, for instance, always result in Huntington’s disease, a degenerative brain condition that usually appears in middle age.
The link between mutations and disease isn’t always so clear-
Certain mutations in the BRCA1 and BRCA2 genes are known genetic risk factors for breast and ovarian cancers, for instance. But not everyone with these mutations develops cancer. According to the National Cancer Institute, about 60% of women with a harmful BRCA1 mutation will develop breast cancer in her lifetime, compared with about 12% of women in the general population. And 15% to 40% of women with a BRCA1 mutation will be diagnosed with ovarian cancer, compared with just 1.4% of women without that genetic change.
The BRCA1 mutations are just some of the thousands of harmful genetic changes that geneticists have identified so far. Other common mutations have been shown to elevate one’s risk of developing heart disease, diabetes, various cancers, and numerous other common illnesses. Often, these mutations involve changes to just a single base pair of DNA. These common single-
245
Genome sequencing technology has improved significantly over the last decade, making it easier and less expensive to scan an individual’s DNA for potentially harmful SNPs. A number of companies now offer genetic tests directly to the public. Unlike tests such as the BRCA1 blood test that Gilmore was given, these direct-
Some of these tests aren’t related to health at all. Your personal genome contains many unique features—
Other popular DTC tests inspect DNA samples for SNPs associated with certain diseases and physical traits—
Advocates of the tests say the technology puts the power of genetic information in the hands of consumers. Critics, on the other hand, argue that the information provided by DTC tests isn’t always very meaningful. The presence of some SNPs might raise the risk of a rare disease by just 2% or 3%, for example. In many cases, the precise link between mutation and disease is still being sorted out. Also, without input from a genetic counselor or medical professional, consumers may not know how to interpret the information revealed by the tests. The American Medical Association has recommended that a doctor always be involved when any genetic testing is performed.
246
Moreover, knowing your genetic risk factors isn’t the whole story. When it comes to your health and well-
Claudia Gilmore is especially careful to exercise regularly and eat a healthy diet. Still, she can only control her environment to a degree. She knew that, given her genetic status, her risk of breast cancer remained high. She made the extraordinary decision to eliminate that risk by undergoing a mastectomy at the age of 23. It wasn’t an easy decision, she says, but she feels privileged to have been able to take proactive steps to protect her health. “I’ll be a ‘previvor’ instead of a survivor,” she says.
Two years later the same medical choice was made by the American actress, film director, screenwriter, and author Angelina Jolie, who recounted her experience in the New York Times, writing, “Once I knew that this [BRCA1 mutation] was my reality, I decided to be proactive and to minimize the risk as much I could. I made a decision to have a preventive double mastectomy. I started with the breasts, as my risk of breast cancer is higher than my risk of ovarian cancer, and the surgery is more complex. . . . I am writing about it now because I hope that other women can benefit from my experience.”1
For now, it’s still too costly to sequence every individual’s entire genome. But each year, many more genetic tests hit the market. Already, doctors are beginning to design medical treatments based on a patient’s personal genome. People with a certain genetic profile, for example, are less likely than others to benefit from statins, medications prescribed to lower cholesterol. Doctors are also choosing which cancer drugs to prescribe based on the unique genetic signatures of patients and their tumors.
We’ve only just entered the era of personal genomics. While there’s much left to decipher, it’s clear that each of our individual genomes contains a wealth of biological information. And, as Gilmore says, “I’ve always been taught that knowledge is power.”
CASE 3 QUESTIONS
Special sections in Chapters 12–
What new technologies are being developed to sequence your personal genome? See page 264.
Why sequence your personal genome? See page 274.
What can your personal genome tell you about your genetic risk factors? See page 294.
How can genetic risk factors be detected? See page 317.
How do genetic tests identify disease risk factors? See page 342.
How can the Y chromosome be used to trace ancestry? See page 358.
How can mitochondrial DNA be used to trace ancestry? See page 360.
Can personalized medicine lead to effective treatments of common diseases? See page 375.
How do lifestyle choices affect expression of your personal genome? See page 386.
Can cells with your personal genome be reprogrammed for new therapies? See page 403.