Pax6 is a master regulator of eye development.
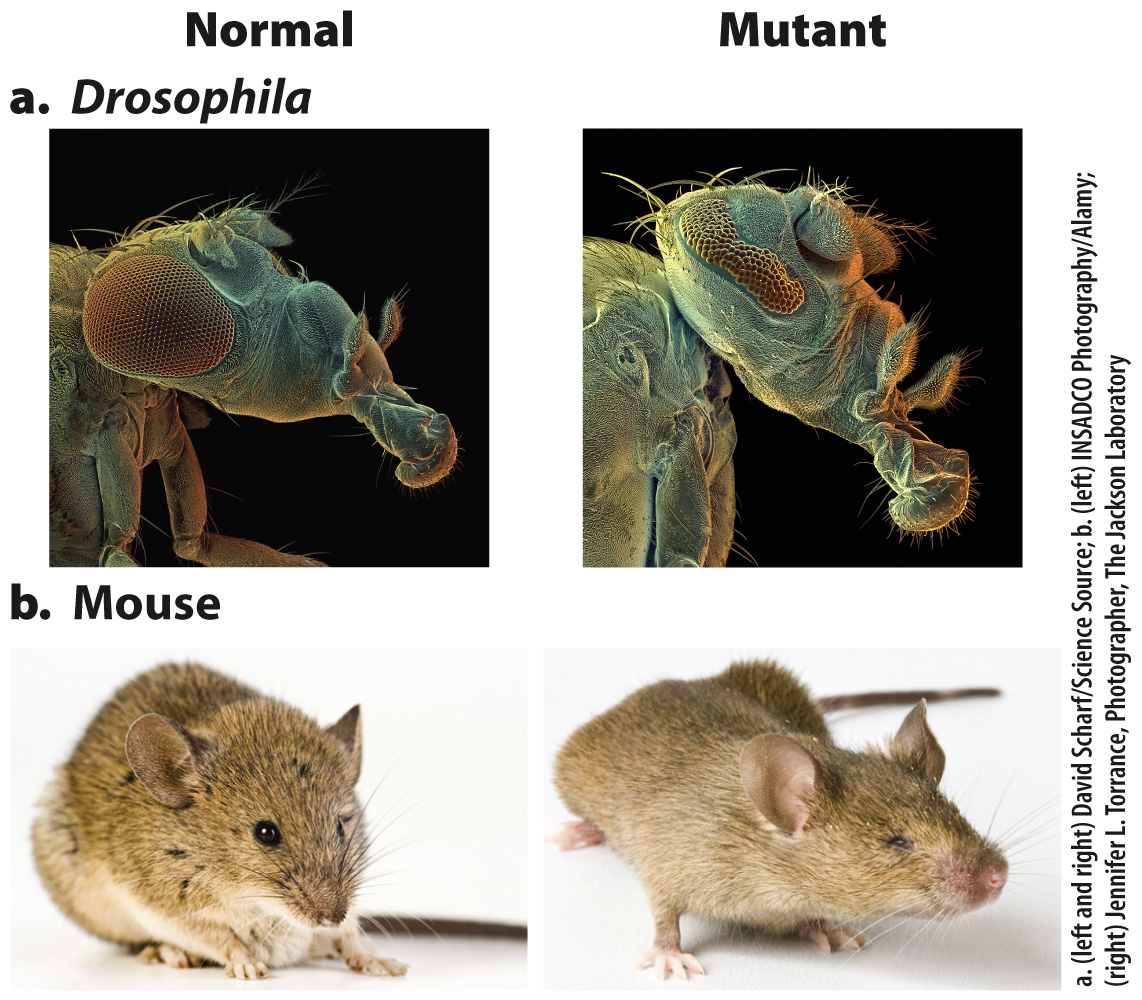
FIG. 20.17 Effect of Pax6 mutations on eye development. (a) Normal and eyeless mutant in Drosophila; (b) normal and small eye mutant in mouse.
The diversity of animal eyes suggests that they may have evolved independently in different organisms. However, more recent evidence suggests an alternative hypothesis—that they evolved once, very early in evolution, and subsequently diverged over time. One piece of evidence that supports the hypothesis of a single origin for light perception is the observation that the light-sensitive molecule in all light-detecting cells is the same, a derivative of vitamin A in association with a protein called opsin (Chapter 36). The presence of the same light-sensitive molecule in diverse eyes argues that it may have been present in the common ancestor of all animals with eyes and has been retained over time.
Another argument against multiple independent origins of eyes came from studies of eye development. Researchers identified eyeless, a gene in the fruit fly Drosophila. As its name implies, the phenotype of eyeless mutants is abnormal eye development (Fig. 20.17a). When the protein product of the eyeless gene was identified, it was found to be a transcription factor called Pax6. Mutant forms of a Pax6 gene were already known to cause small eyes in the mouse (Fig. 20.17b) and aniridia (absence of the iris) in humans.
The mutations in the Pax6 gene that cause the development defects in the eye in fruit flies, mice, and humans are loss-of-function mutations. A loss-of-function mutation is just that: a mutation that inactivates the normal function of a gene. In this case, loss-of-function mutations in Pax6 make a defective version of the Pax6 transcription factor that is not able to carry out its function.
The similarity in phenotypes of Pax6 mutants, along with the strong conservation of Pax6 in Drosophila and mouse, led Swiss developmental biologist Walter Gehring to hypothesize that Pax6 might be a master regulator of eye development. In other words, he hypothesized that Pax6 binds to regulatory regions of a set of genes that turns on a developmental program that induces eye development. In theory, this means that Pax6 can induce the development of an eye in any tissue in which it is expressed.
To test this hypothesis, Gehring and collaborators genetically engineered fruit flies that would produce the normal Pax6 transcription factor in the antenna, where it is not normally expressed (Fig. 20.18a). Any mutation in which a gene is expressed in the wrong place or at the wrong time is known as a gain-of-function mutation. (We saw an example of gain-of-function mutations when the Hox genes were expressed in the wrong segments of the fruit fly, producing legs where antennae normally develop and thus resulting in four-winged fruit flies.) For genes that control a developmental pathway, loss-of-function mutations and gain-of-function mutations often have opposite effects on phenotype.
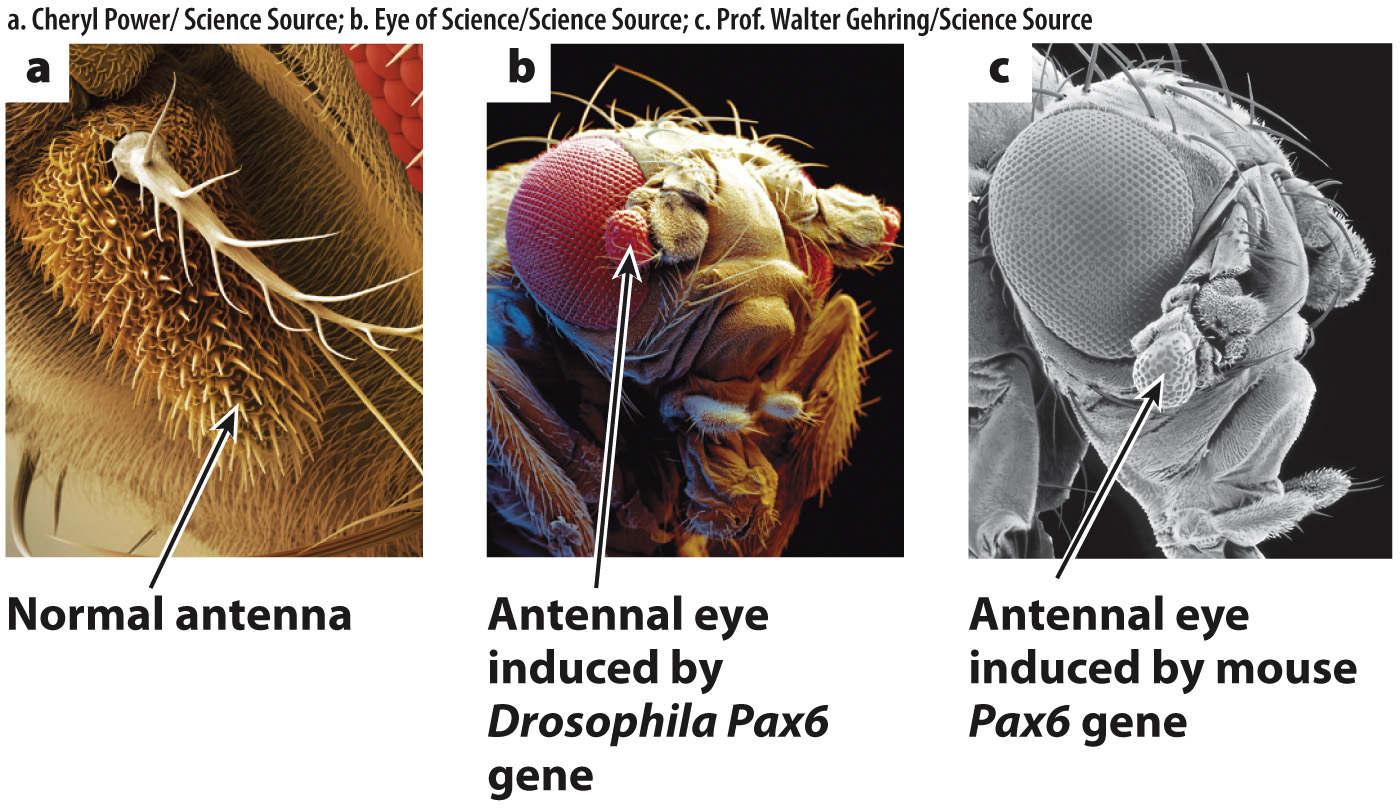
FIG. 20.18 Pax6, a master switch controlling eye development. (a) Normal fly antenna; (b) eye tissue induced by expression of fruit fly Pax6 in the antenna; (c) eye tissue induced by expression of mouse Pax6 in the antenna.
Quick Check 5 For genes that control pathways of development, loss-of-function mutations are usually recessive whereas gain-of-function mutations are usually dominant. Can you suggest a reason why?
Quick Check 5 Answer
Sexually reproducing organisms carry two copies of most genes, one from the mother and the other from the father. If either of these is knocked out by a loss-of-function mutation, the other is still present and compensates for the mutant. Hence, a loss-of-function mutation is expected to be recessive. In a gain-of-function mutation, one of the gene copies is expressed in the wrong amount, or the wrong tissue, or at the wrong time, and if such a gene turns on a developmental pathway, expression of only one copy of the gene is sufficient to turn the pathway on. Hence, a gain-of-function mutation in a gene that controls a developmental pathway is expected to be dominant.
Since loss-of-function mutations in Pax6 result in an eyeless phenotype, a gain-of-function mutation should result in eyes developing in whatever tissue Pax6 is expressed. In the gain-of-function mutation, the antenna developed into a miniature compound eye, and electrical recordings demonstrated that some of these antennal eyes were functional (Fig. 20.18b). The researchers also created other gain-of-function mutations that led to eyes on the legs, wings, and other tissues, which the New York Times publicized in an article headlined “With New Fly, Science Outdoes Hollywood.”
Gehring and his group then went one step further. They took the Pax6 gene from mice and expressed it in fruit flies to see whether the mouse Pax6 gene is similar enough to the fruit fly version of the gene that it could induce eye development in the fruit fly. Specifically, they created transgenic fruit flies that expressed the Pax6 gene from mice in the fruit fly antenna. The mouse gene induced a miniature eye in the fly (Fig. 20.18c). Note, however, that the Pax6 gene from mice induced the development of a compound eye of Drosophila, not the single-lens eye of mouse.
The ability of mouse Pax6 to make an eye in fruit flies suggests that mouse and fruit fly Pax6 are not only similar in DNA and amino acid sequence, but also similar in function, and indeed act as a master switch that can turn on a developmental program leading to the formation of an eye. But these observations lead to another question: Why did the mouse Pax6 gene produce fruit fly eyes instead of mouse eyes?
The answer is that the fruit fly genome does not include the genes needed to make mouse eyes. Mouse Pax6 protein induces fly eyes because of the downstream genes affected by Pax6, those that function later in the process of eye development. Transcription factors like Pax6 interact with their target genes by binding with short DNA sequences adjacent to the gene, usually at the 5' end, called cis-regulatory elements. These regulatory elements are located in the promoter and help determine whether the adjacent DNA is transcribed. When bound to cis-regulatory elements, some transcription factors act as repressors that prevent transcription of the target gene, and others serve as activators by recruiting the transcriptional machinery to the target gene (Chapter 19). A transcription factor can even repress some of its target genes and activate others.
In the fruit fly, Pax6 binds to cis-regulatory elements in many genes, turning some genes on and others off. The products of these downstream genes in turn affect the expression of further downstream genes. The total number of genes that are downstream of Pax6 and that are needed for eye development is estimated at about 2000. Most are not direct targets of Pax6 but are activated indirectly through other transcription factors downstream of Pax6. When mouse Pax6 is expressed in fruit flies, it is similar enough in sequence to activate the genes involved in fruit fly eye development, so it makes sense that mouse Pax6 leads to the development of a fruit fly eye, not a mouse eye.
One scenario of how Pax6 became a master switch for eye development in a wide range of organisms, but produces a diversity of eyes in these organisms, is that Pax6 evolved early in the history of life as a transcription factor able to bind to and regulate genes involved in early eye development. Over time, different genes in different organisms acquired new Pax6-binding cis-regulatory elements by mutation, and if these were beneficial they persisted. The downstream genes that are targets of Pax6 therefore are different in different organisms, but they share two features—they are regulated by Pax6 and they are involved in eye development. So, the early steps are conserved, but the later ones are not.
The Pax6 gene and the Hox genes reveal an important principle in evo-devo, which is that master regulatory genes that control development are often evolutionarily conserved, whereas the downstream genes that they regulate may not be. Downstream genes can evolve new functions, or genes not originally controlled by the master regulator may evolve to come under its influence, or genes formerly controlled by the master regulator may evolve to be unresponsive. In this way, a conserved master regulatory mechanism may result in distinct developmental outcomes in different organisms. In the case of Pax6, for example, the master regulatory mechanism for eye development evolved early and is shared among a wide range of animals, even though the eyes that are produced are quite diverse.

