Natural selection can be stabilizing, directional, or disruptive.
Up to this point, we have followed the fate of individual mutations, which can increase, decrease, or be maintained at an intermediate frequency under the influence of natural selection. We can also look at the consequence of natural selection from a different perspective. Instead of following individual mutations, we can look at changes over time of a particular trait of an organism. For example, we might track the evolution of height in a population, despite not knowing the specifics of the genetic basis of height differences. When we look at natural selection from this perspective, we see three types of patterns: stabilizing, directional, and disruptive.
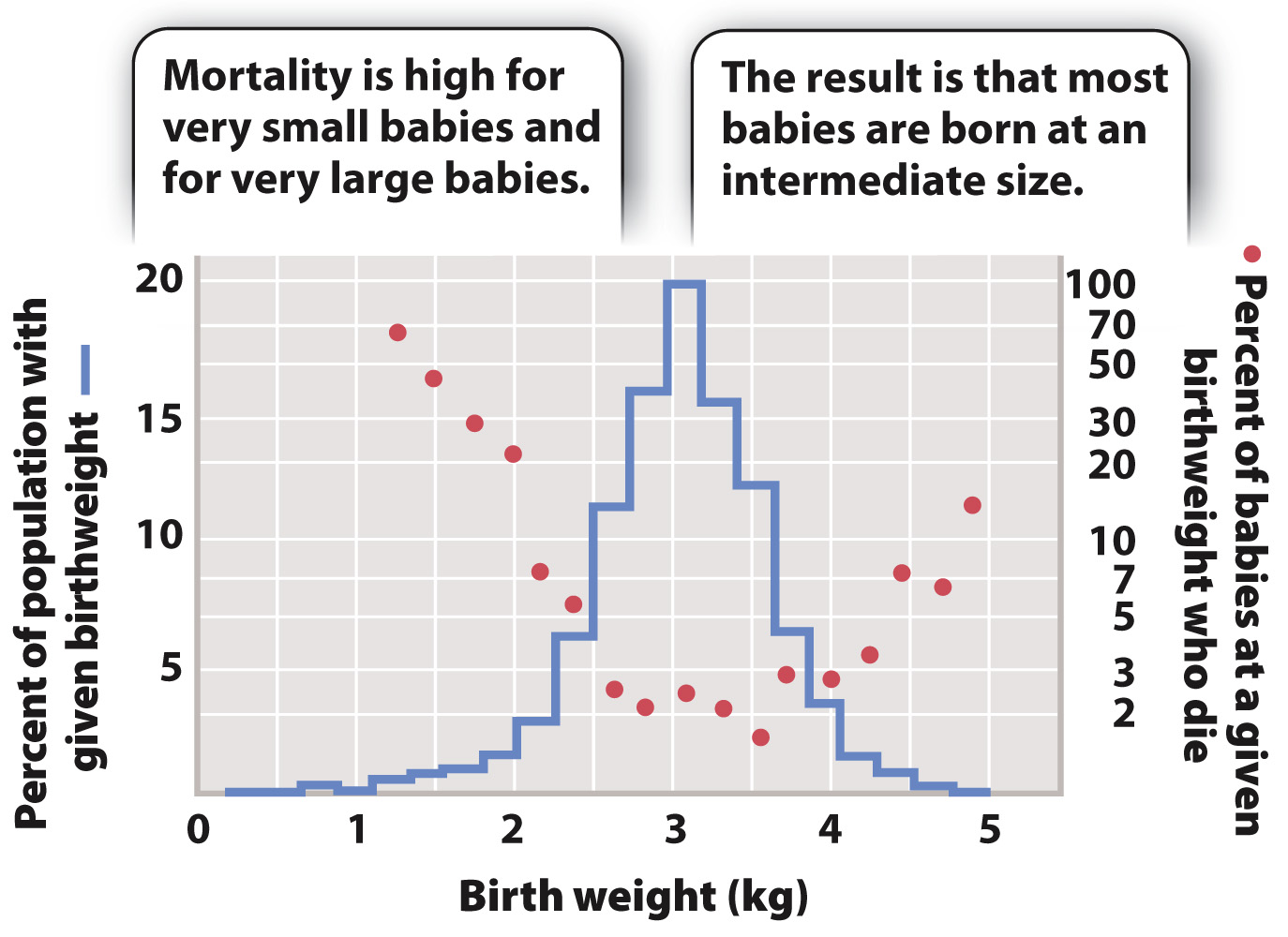
Stabilizing selection maintains the status quo and acts against extremes. A good example is provided by human birth weight, a trait affected by a number of factors, including many fetal genes (Fig. 21.8). If a baby is too small, then its chances of survival after birth are low. However, if it is too big, there may be complications during delivery that endanger both mother and baby. Thus, the optimum birth weight is between these two extremes. In this case, natural selection acts against the extremes. The vast majority of natural selection is of this kind as deleterious mutations that cause a departure from the optimal phenotype are selected against.
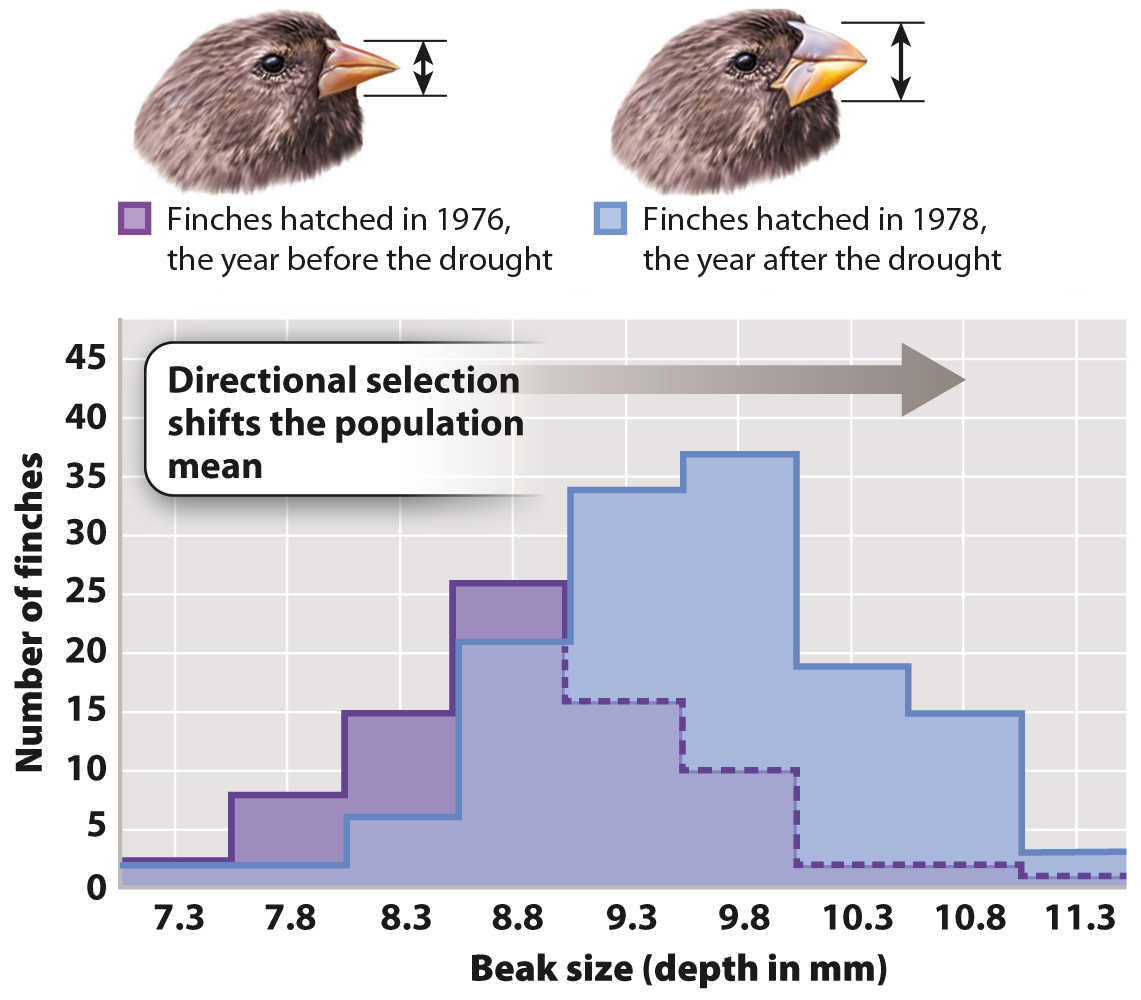
Whereas stabilizing selection keeps a trait the same over time, directional selection leads to a change in a trait over time. A well-
436
Artificial selection, which has been practiced by humans since at least the dawn of agriculture, is a form of directional selection. Artificial selection is analogous to natural selection, but the competitive element is removed. Successful genotypes are selected by the breeder, not through competition. Because it can be carefully controlled by the breeder, artificial selection is astonishingly efficient at generating genetic change. Practiced over many generations, artificial selection can create a population in which the selected phenotype is far removed from that of the starting population. Fig. 21.10 shows the result of long-
HOW DO WE KNOW?
FIG. 21.10
How far can artificial selection be taken?
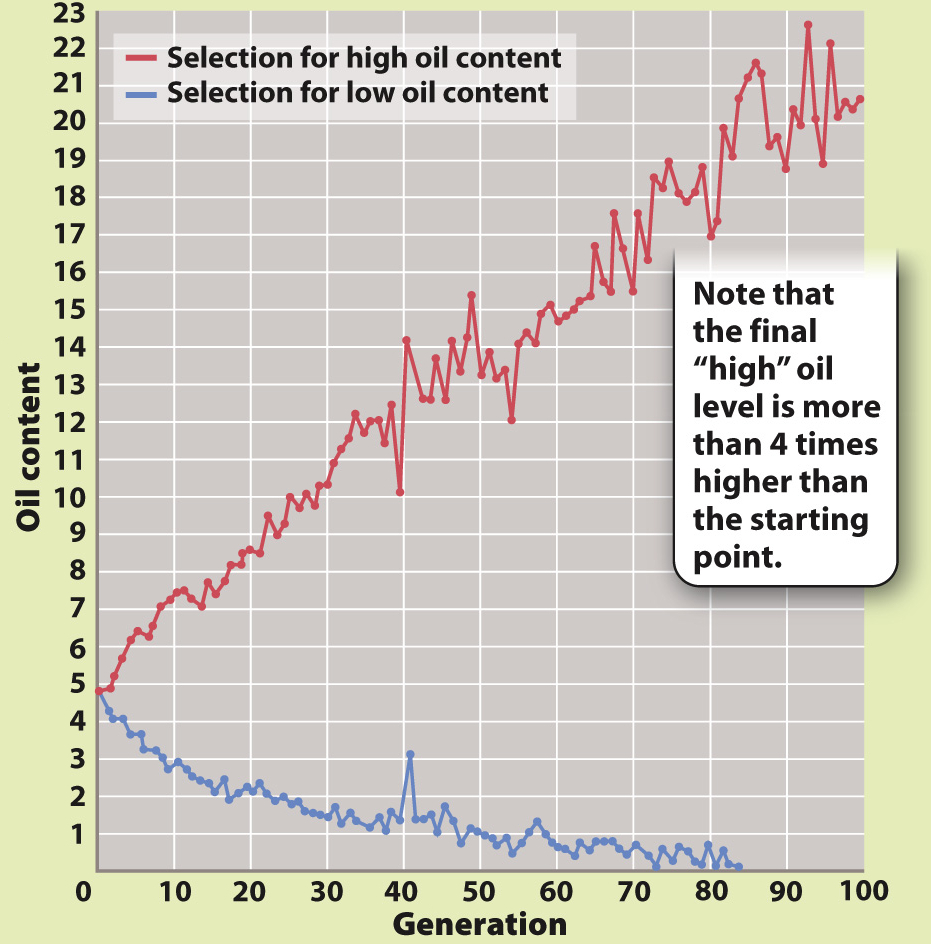
BACKGROUND Begun in the 1890s and continuing to this day at the University of Illinois as an attempt to manipulate the properties of corn, this experiment has become one of the longest-
HYPOTHESIS Researchers hypothesized that there is a limit to the extent to which a population can respond to continued directional selection.
EXPERIMENT Corn was artificially selected for either high oil content or low oil content: Every generation, researchers bred together just the plants that produced corn with the highest oil content, and did the same for the plants that produced corn with the lowest oil content. Every generation, kernels showed a range of oil levels, but only the 12 kernels with the highest or the lowest oil content were used for the next generation.
RESULTS In the line selected for high oil content, the percentage of oil more than quadrupled, from about 5% to more than 20%. In the line selected for low oil content, the oil content fell so close to zero that it could no longer be measured accurately, and the selection was terminated. Both selected lines are completely outside the range of any phenotype observed at the beginning of the experiment.
FOLLOW-
SOURCE Moose, S. P., J. W. Dudley, and T. R. Rocheford. 2004. “Maize Selection Passes the Century Mark: A Unique Resource for 21st Century Genomics.” Trends in Plant Science 9:358–
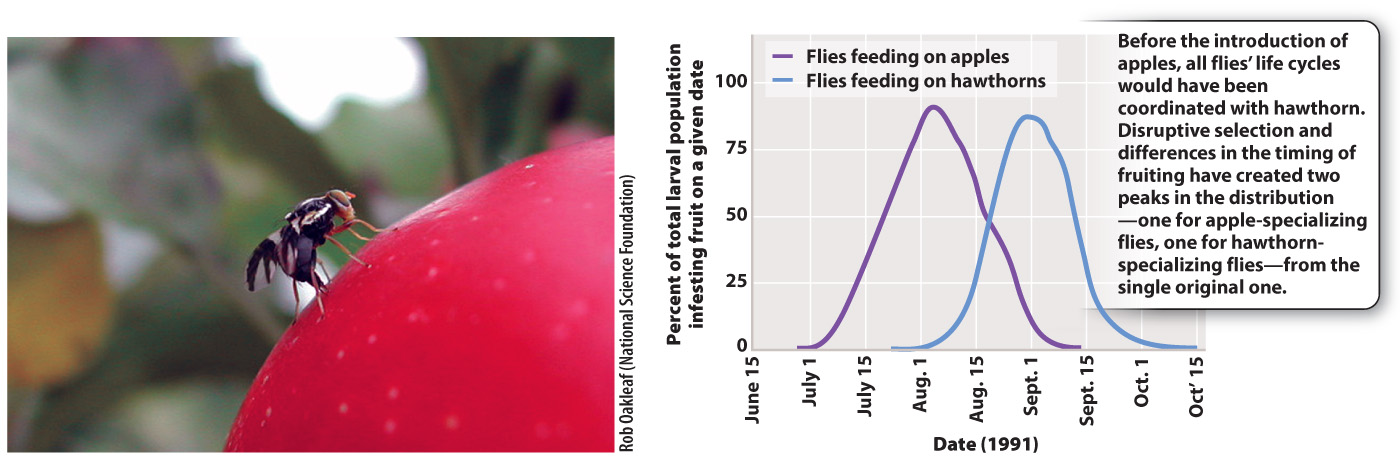
A third mode of selection, known as disruptive selection, operates in favor of extremes and against intermediate forms. Apple maggot flies of North America, Rhagotletis pomonella, provide an example (Fig. 21.11). The larvae of these flies feed on the fruit of hawthorn trees. However, with the introduction of apples from Europe about 150 years ago, these flies have become pests of apples. Apple trees flower and produce fruit earlier every summer than hawthorns, so disruptive selection has resulted in the production of two genetically distinct groups of flies, one specializing on apple trees and the other on hawthorn trees. Disruptive selection acts against intermediates between the two groups, which miss the peaks of both the apple and hawthorn seasons. We explore this mechanism, which can lead to the evolution of new species, in more detail in the next chapter.
437