Dispersal and vicariance can isolate populations from each other.
How do populations become allopatric? There are two ways. The first is by dispersal, in which some individuals colonize a distant place, such as an island, far from the main source population. The second is by vicariance, in which a geographic barrier arises within a single population, separating it into two or more isolated populations. For example, when sea levels rose at the end of the most recent ice age, new islands formed along the coastline as the low-
452
453
Regardless of how the allopatric populations came about—
Often, vicariance-
HOW DO WE KNOW?
FIG. 22.7
Can vicariance cause speciation?
BACKGROUND Three and a half million years ago, the Isthmus of Panama was not completely formed. Several marine corridors remained open, allowing interbreeding between marine populations in the Caribbean and the eastern Pacific. Subsequently, the gaps in the isthmus were plugged, separating the Caribbean and eastern Pacific populations and preventing gene flow between them.
HYPOTHESIS Nancy Knowlton and her colleagues hypothesized that patterns of speciation would reflect the impact of the vicariance resulting from the closing of the direct marine connections between the Pacific and the Caribbean. Specifically, they predicted that each ancestor species (from the time before the formation of the isthmus) split into two “daughter” species, one in the Caribbean and the other in the Pacific. The closest relative of each current Pacific species, then, is predicted to be a Caribbean species (and vice versa).
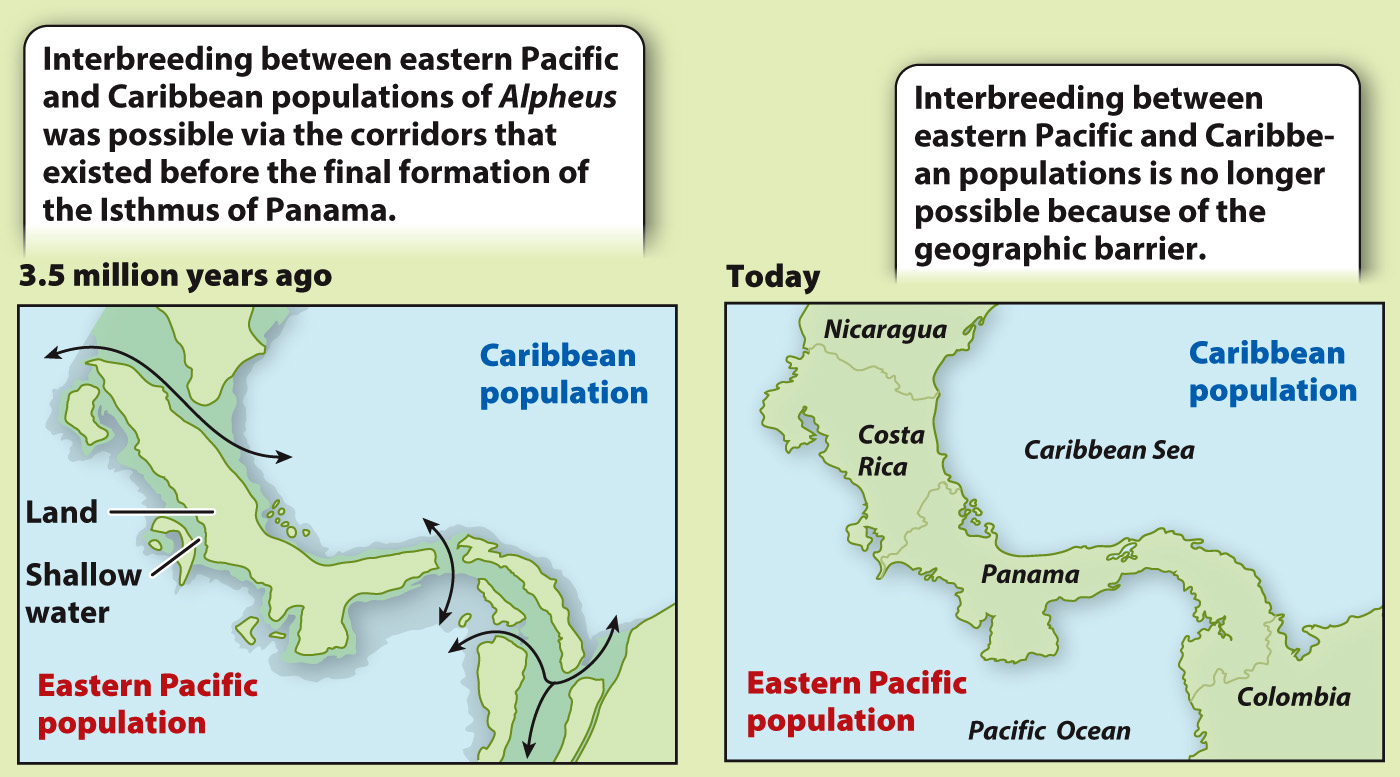
EXPERIMENT This study focused on 17 species of snapping shrimp in the genus Alpheus, a group that is distributed on both sides of the isthmus. The first step was to sequence the same segment of DNA from each species. The next step was to compare those sequences in order to reconstruct the phylogenetic relationships among the species.
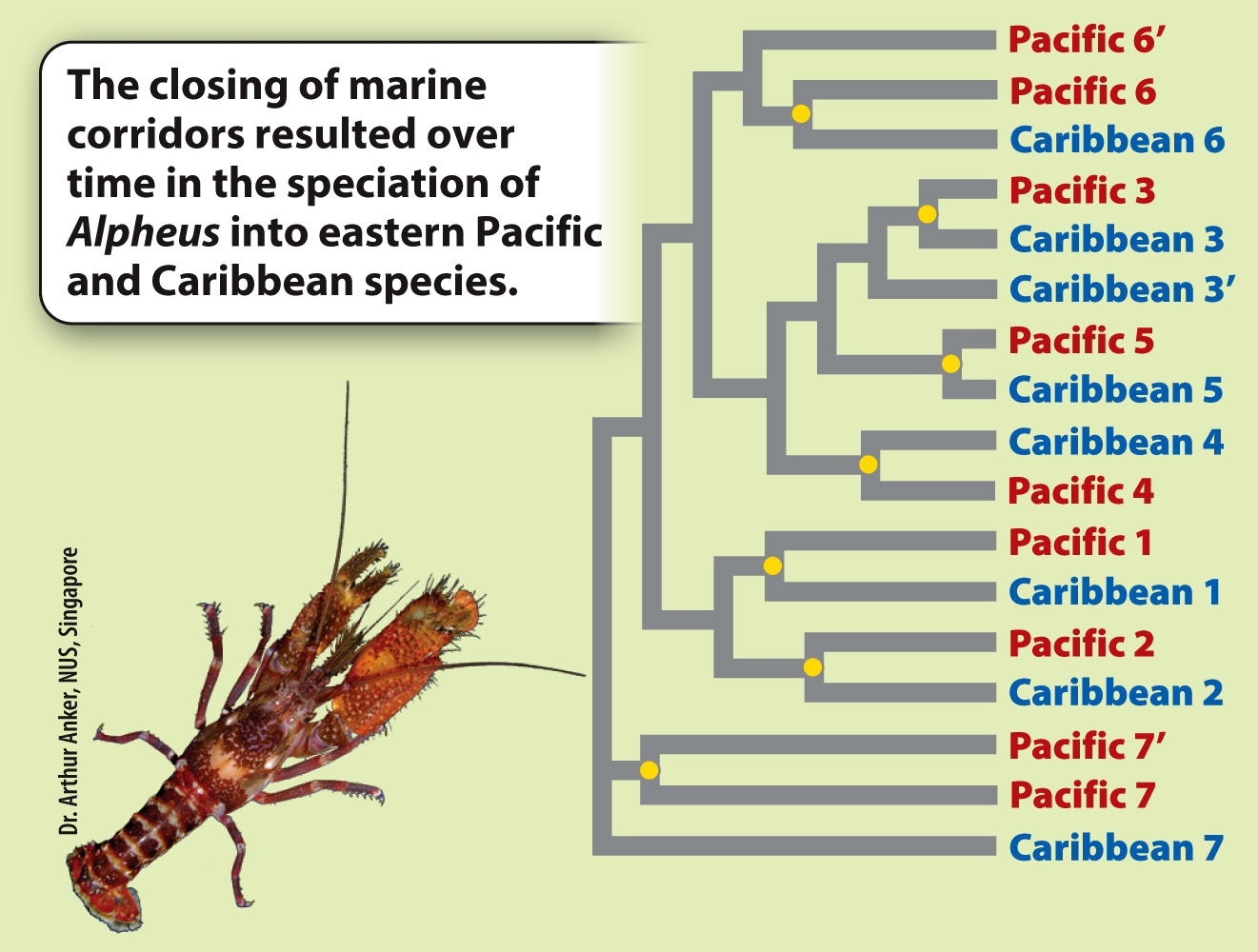
RESULTS The phylogeny reveals that species show a distinctly paired pattern of relatedness: The closest relative of each species is one from the other side of the isthmus.
CONCLUSION That we see these consistent Pacific/Caribbean sister species pairings strongly supports the hypothesis that the vicariance caused by the formation of the isthmus has driven speciation in Alpheus. Each Pacific/Caribbean pairing is derived from a single ancestral species (indicated by a yellow dot) whose continuous distribution between the Caribbean and eastern Pacific was disrupted by the formation of the isthmus. Here, we see striking evidence of the role of vicariance in multiple speciation events.
FOLLOW-
SOURCE Knowlton, N., et al. 1993. “Divergence in Proteins, Mitochondrial DNA, and Reproductive Compatibility Across the Isthmus of Panama.” Science 260 (5114):1629–
Dispersal is important in a specific kind of allopatric speciation known as peripatric speciation (that is, in a peripheral place). In this model, a few individuals from a mainland population (the central population of a species) disperse to a new location remote from the original population and evolve separately. This may be an intentional act of dispersal, such as young mammals migrating away from where they were raised, or it could be an accident brought about by, for example, an unusual storm that blows migrating birds off their normal route. The result is a distant, isolated island population. “Island” in this case may refer to a true island—
The island population is classically small and often in an environment that is slightly different from that of the mainland population. The peripatric speciation model suggests that change accumulates faster in these peripheral isolated populations than in the large mainland populations, both because genetic drift is more pronounced in smaller populations than in larger ones and because the environment may differ between the mainland and island in a way that results in natural selection driving differences between the two populations. These mechanisms cause genetic divergence of the island population from the mainland one, ultimately leading to speciation.
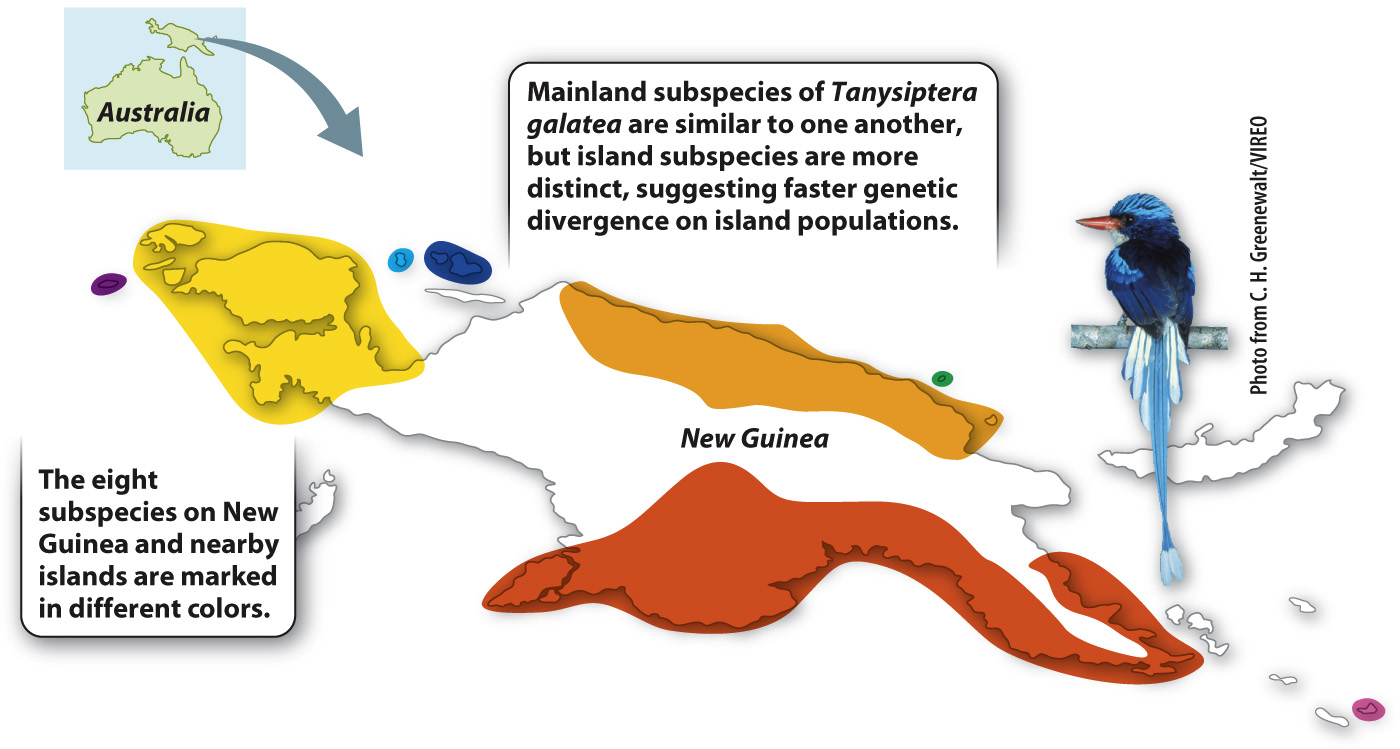
It is possible to glimpse peripatric differentiation in action (Fig. 22.8). Studies of a kingfisher, Tanysiptera galatea, in New Guinea and nearby islands show the process under way. There are eight recognized subspecies of T. galatea, three on mainland New Guinea (where they exist in large populations separated by mountain ranges) and five on nearby islands (where, because the islands are small, the populations are correspondingly small). The mainland subspecies are still quite similar to one another, but the island subspecies are much more distinct, suggesting that genetic divergence is occurring faster in the small island populations. If we wait long enough, these subspecies will probably diverge into new species.
454
455
Because dating such dispersal events is tricky—
The finches’ subsequent dispersal among the other islands of the Galápagos has promoted further peripatric speciation (Fig. 22.9). In the graph at the bottom of the figure, we see that the number of finch species is correlated with the number of islands in the archipelago. This is clear indication of the importance of geographic separation (allopatry) in speciation: The availability of islands provided opportunities for populations to become isolated from one another, allowing speciation. The result was the evolution of 13 different species of finches, collectively known today as Darwin’s finches.
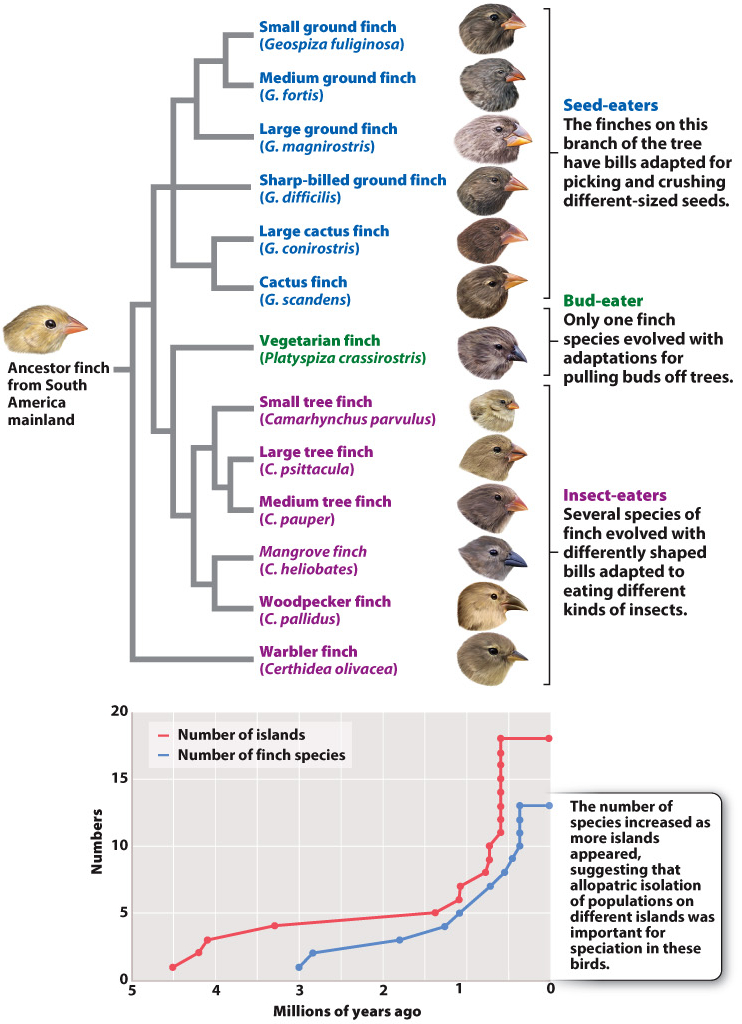
The Galápagos finches and their frenzy of speciation illustrate the important evolutionary idea of adaptive radiation, a bout of unusually rapid evolutionary diversification in which natural selection accelerates the rates of both speciation and adaptation. Adaptive radiation occurs when there are many ecological opportunities available for exploitation. Consider the ancestral finch immigrants arriving on the Galápagos. A wealth of ecological opportunities was open and available. Until the arrival of the colonizing finches, there were no birds on the islands to eat the plant seeds, or to eat the insects on the plants, and so on. Suppose that the ancestral finches fed specifically on medium-
When the medium-
Quick Check 2 Why do we see so many wonderful examples of adaptive radiation on mid-
Quick Check 2 Answer
Volcanic island chains offer many examples of adaptive radiation because they are dependent on the serendipitous process of colonization. This means that only some plants and animals are able to get there. In addition, because of the absence of competitors on these islands, there are often many available ecological opportunities for the colonizers. For example, there may be no insect-