Carbon isotopes show that much of the CO2 added to air over the past half century comes from burning fossil fuels.
We can test the hypothesis that human activities have contributed to the increases in atmospheric CO2 measured at Mauna Loa and in ice cores by making careful measurements of a chemical detail: the isotopic composition of atmospheric CO2. In Chapter 2, we saw that many elements have several isotopes, atoms of the element that vary in atomic mass because they have different numbers of neutrons. Carbon has three isotopes: 12C (with six neutrons, about 99% of all carbon atoms); 13C (with seven neutrons, most of the remaining 1%); and the rare 14C (with eight neutrons, about one part per trillion of atmospheric carbon).
More than 40 years ago, the Austrian-
HOW DO WE KNOW?
FIG. 25.4
What is the major source of the CO2 that has accumulated in Earth’s atmosphere over the past two centuries?
BACKGROUND Chemical analyses show that CO2 levels in the current atmosphere are about 40% higher than they were at the time of the American Revolution. This rise coincides with major advances in manufacturing and transportation, which are driven by the burning of fossil fuels. These coincidences in timing suggest that human activities are responsible for increasing CO2 levels.
HYPOTHESIS The burning of fossil fuels, central to the emergence of modern industrial societies, has been the principal cause of the measured increase of CO2 in the atmosphere.
EXPERIMENT AND RESULTS Hans Suess measured the abundance of the two stable isotopes of carbon, 13C and 12C, in air samples and demonstrated that the ratio of 13C to 12C in atmospheric CO2 has decreased over the second half of the twentieth century. Additional measurements of coral skeletons and wood show that the ratio of 13C to 12C in air has been decreasing over the entire period during which atmospheric CO2 levels have been increasing.
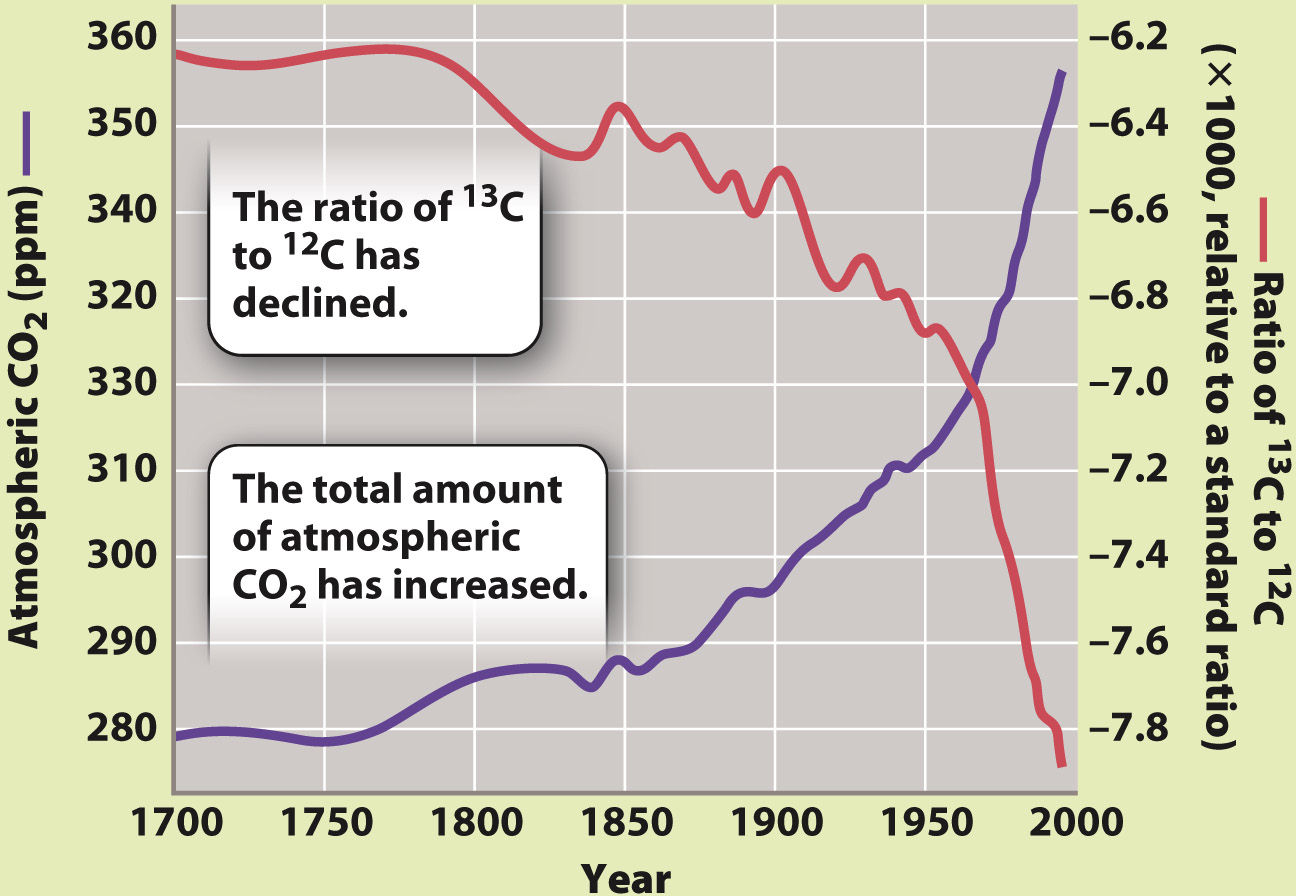
DISCUSSION The ratio of 13C to 12C in CO2 added to the atmosphere over the past 200 years is lower than that in CO2 that was already in the air more than 200 years ago. The ratio of 13C to12C in CO2 emitted by volcanoes is too high to account for the data, as is the ratio in CO2 released from the oceans. In contrast, organic matter formed by photosynthesis has just the right ratio of 13C to 12C to account for Suess’s measurements. By itself, the changing abundance of 13C in the air could reflect the conversion of plant carbon to CO2 by burning, or it could reflect the burning of fossil fuels formed by the burial of plant and algal materials in the geologic past.
FOLLOW-
CONCLUSION Fossil fuel burning by industrialized societies has been, and continues to be, a principal source of CO2 buildup in Earth’s atmosphere.
SOURCES Revelle, R., and H. E. Suess. 1957. “Carbon dioxide exchange between atmosphere and ocean and the question of an increase of atmospheric CO2 during the past decades.” Tellus 9:18–
How does this result influence the hypotheses we developed to account for increasing atmospheric CO2 levels? In photosynthesis, CO2 containing the lighter isotope 12C is incorporated into biomolecules preferentially over CO2 containing 13C, and for this reason organic matter generated by photosynthesis (and the organic matter of organisms that eat photosynthetic organisms) differs in its proportions of 13C and 12C from CO2 from volcanic gases and inorganic carbon dissolved in the oceans. Volcanic and dissolved marine carbon do not have the right ratio of 13C to 12C to explain the isotopic change observed in the atmosphere over time. In contrast, organic matter in living organisms has just the right ratio of 13C to 12C to account for Suess’s measurements. Therefore, processes that cause a net conversion of organic matter to CO2, for example by burning, must be adding CO2 to the atmosphere.
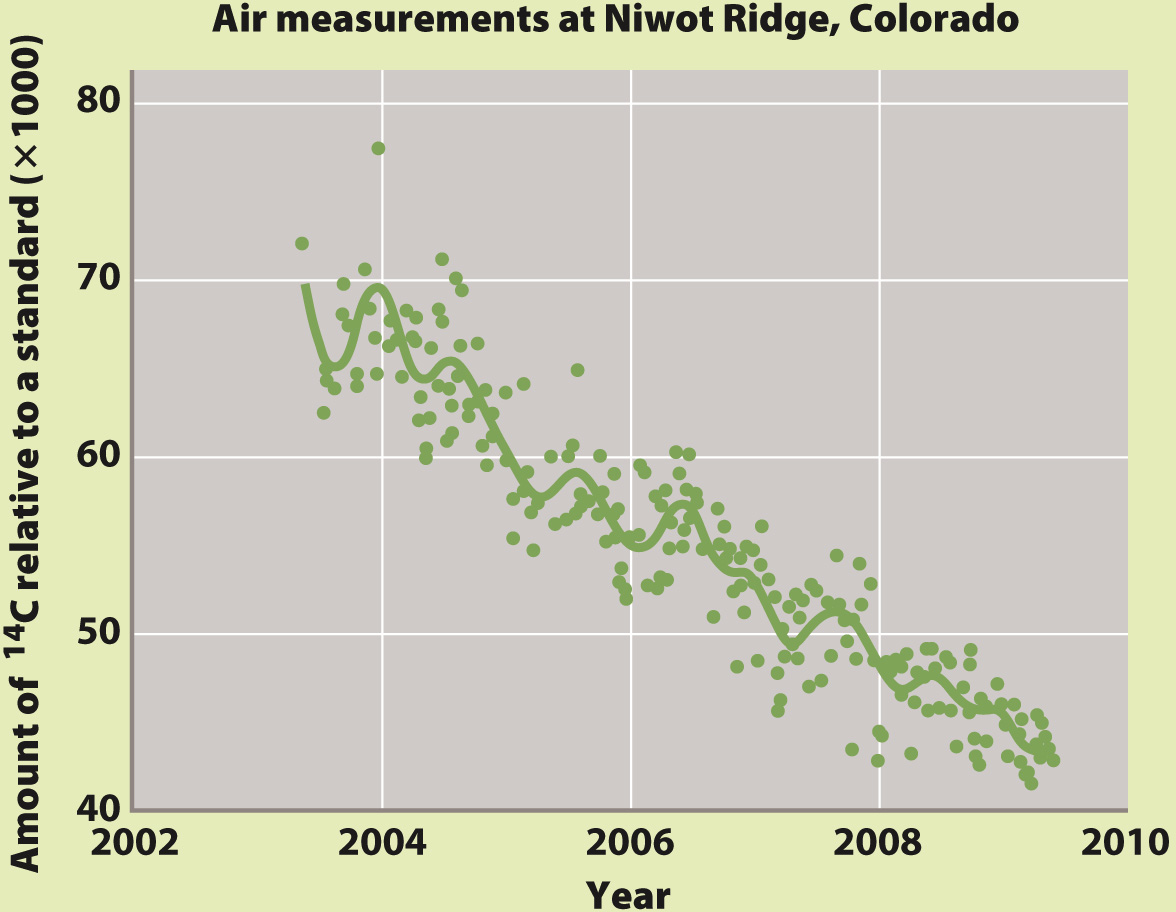
Here’s where the other isotope of carbon, 14C, comes in. Measurements also show that the sources of added CO2 are depleted in 14C relative to the CO2 already in the air. Modern organic matter contains too much 14C to account for the observed pattern, but ancient organic matter—
That atmospheric CO2 is increasing is not a hypothesis; it is a measurement. That the burning of fossil fuels plays an important role in this increase is also unambiguously documented by chemical analyses. Current debate focuses not on these observations, but instead on the more difficult problem of understanding how increasing CO2 will affect climate. We discuss this debate in Chapter 49. For now, however, we can use some relevant numbers to reveal another important aspect of the carbon cycle.
514
515
As shown in the top graph in Fig. 25.5, humans now add almost 9 billion metric tons of carbon to the atmosphere each year, nearly all of it as CO2. Much of this material comes from power plants, airplanes, ships, and automobiles, which oxidize ancient organic matter to CO2 on a grand scale. Further important inputs come from the conversion of forests to agriculture or pastureland. Clearing and burning forest trees also oxidizes organic carbon to CO2.
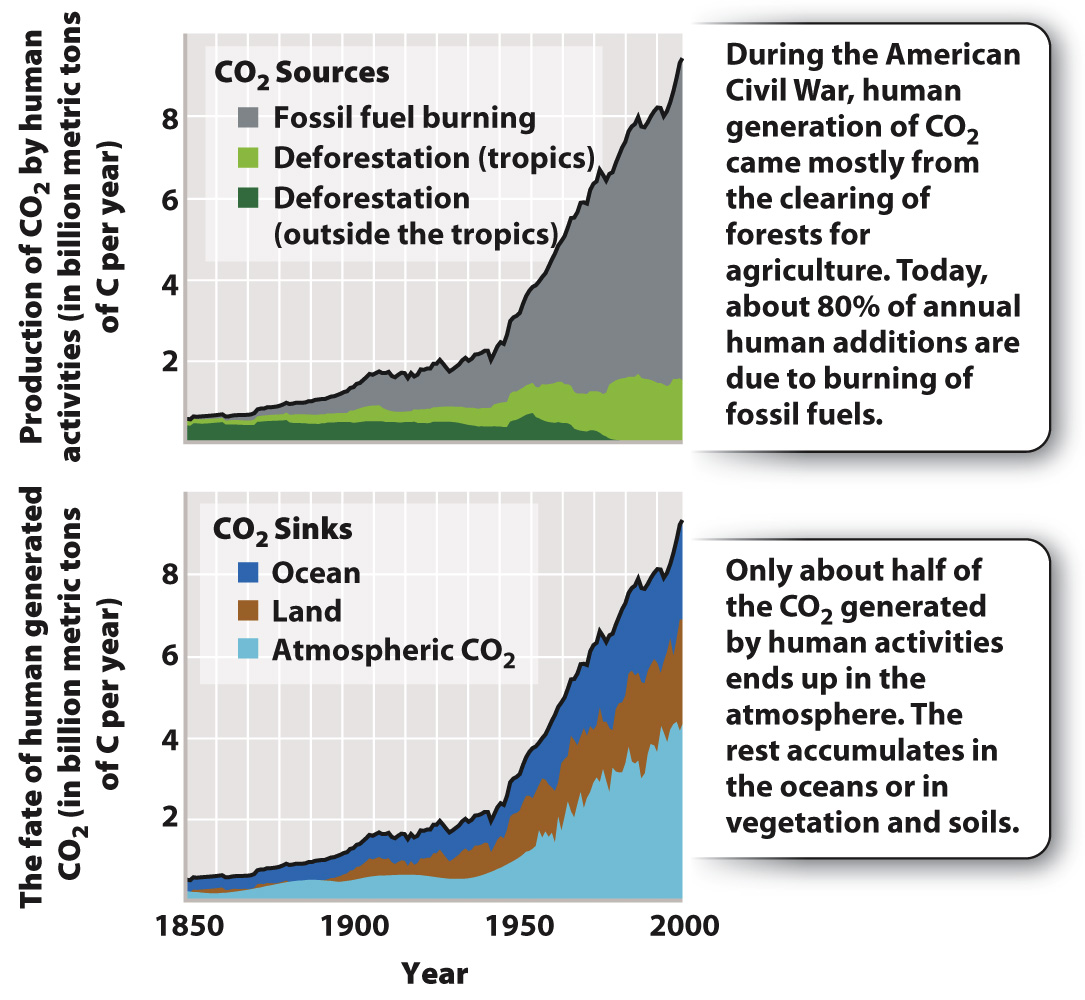
Interestingly, if we compare our best estimates of human CO2 generation since 1958 (not including CO2 added through respiration) with the measured increase in atmospheric CO2, we find that about half of the CO2 released by human activities did not end up in the air. Where did it go? This has been a major question for years, and it now appears that much of the “missing carbon” has been stored as inorganic carbon in the form of CO2, bicarbonate (HCO3–), and carbonate (CO32–