Case 5: What role did symbiosis play in the origin of mitochondria?
CASE 5 THE HUMAN MICROBIOME: DIVERSITY WITHIN
Like chloroplasts, mitochondria closely resemble free-living bacteria in organization and biochemistry. And like chloroplasts, mitochondria contain DNA that confirms their close phylogenetic relationship to a form of bacteria—in this case proteobacteria (Chapter 26). Like chloroplasts, then, mitochondria originated as endosymbiotic bacteria.
Mitochondria are also like chloroplasts in having a small genome. Indeed, the mitochondrial genome is dramatically reduced compared to the ancestral proteobacterial genome, in most eukaryotes containing only a handful of functioning genes. Human mitochondria, for example, code for just 13 proteins and 24 RNAs. Once again, many genes from the original bacterial endosymbiont migrated to the nucleus, where they still reside.
Most eukaryotic cells contain mitochondria, but a few single-celled eukaryotic organisms found in oxygen-free environments do not. Biologists earlier hypothesized that these eukaryotes evolved before the endosymbiotic event that established mitochondria in cells having a nucleus, but that proposal turns out to be wrong. We know this because of the propensity for genes to migrate from the endosymbiont to the host’s nucleus. Every mitochondria-free eukaryote examined to date has relic mitochondrial genes in its nuclear genome, providing support for a second hypothesis, that eukaryotic cells without mitochondria had them once but have lost them.
In fact, many eukaryotes that lack mitochondria contain small organelles called hydrogenosomes that generate ATP by anaerobic processes (Fig. 27.6). These organelles have little or no DNA, but genes of mitochondrial origin in the cells’ nuclei code for proteins that function in the hydrogenosome. Thus, hydrogenosomes appear to be highly altered mitochondria adapted to life in oxygen-poor environments. Other anaerobic eukaryotes contain still smaller organelles called mitosomes that also appear to be remnant mitochondria. Interestingly, mitosomes have lost all capacity for metabolism, retaining only genes needed for assembling the clusters of iron and sulfur found in many proteins. Why these genes are retained in a modified mitochondrion remains unknown.
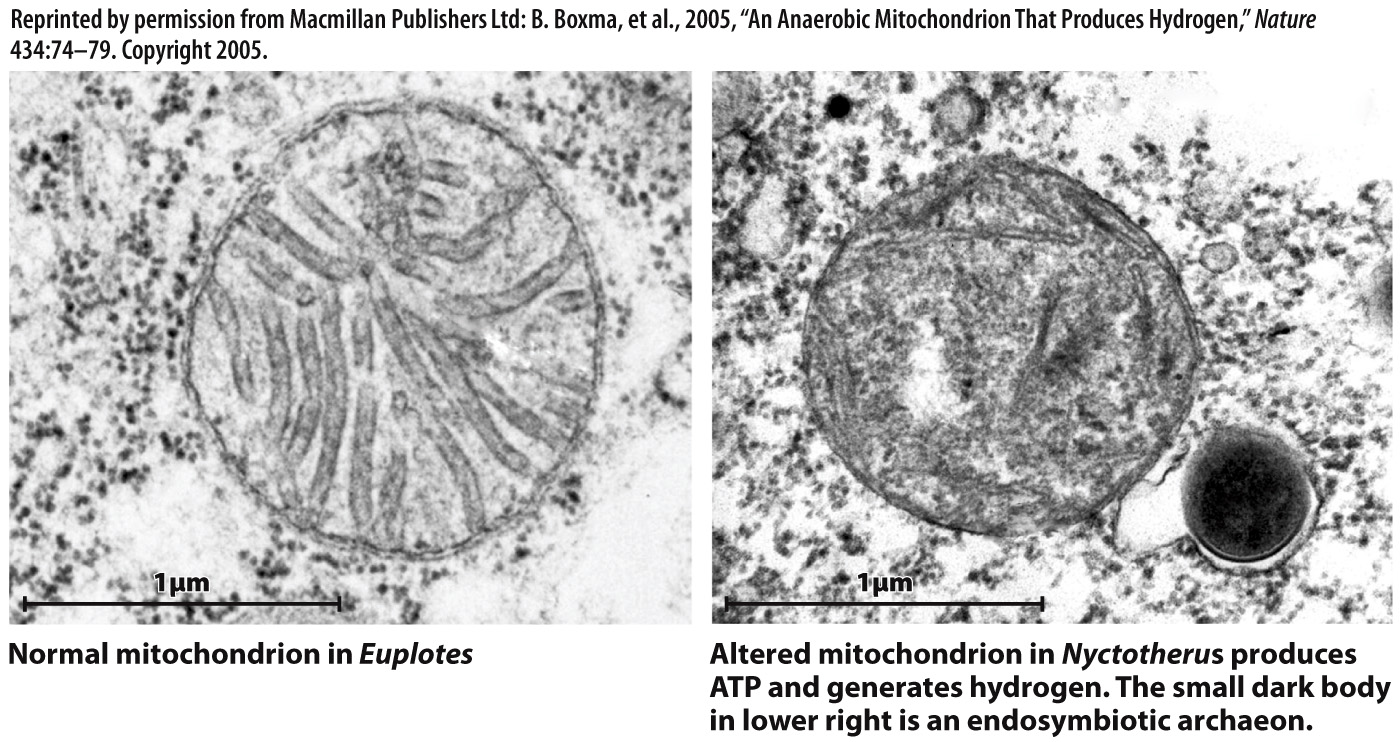
FIG. 27.6 Transmission electron microscope images comparing mitochondria within the aerobic protist Euplotes (left) and Nyctotherus, a close relative that lives in anoxic environments (right). The organelle in Nyctotherus is halfway between a normal mitochondrion and a hydrogenosome, supporting the view that these two organelles are descended from a common ancestor.
