Hydrogen bonds give water many unusual properties.
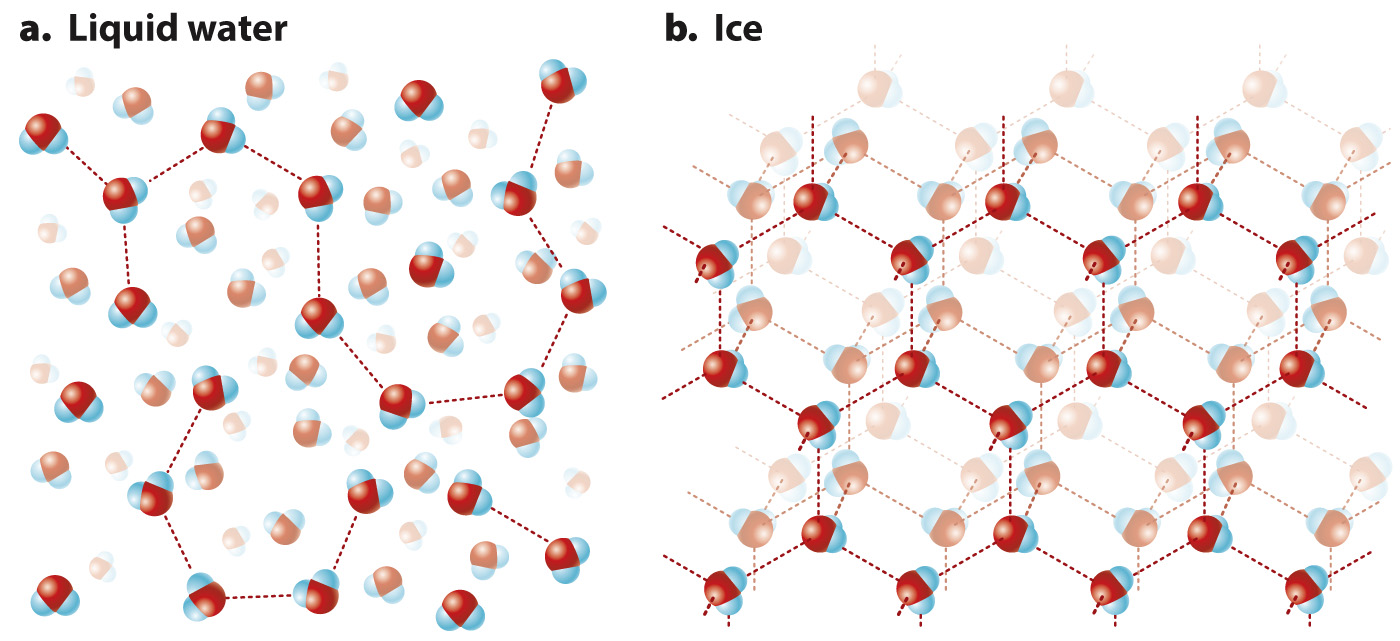
Hydrogen bonds influence the structure of both liquid water (Fig. 2.11a) and ice (Fig. 2.11b). When water freezes, most water molecules become hydrogen bonded to four other water molecules, forming an open crystalline structure we call ice. As the temperature increases and the ice melts, some of the hydrogen bonds are destabilized and break, allowing the water molecules to pack more closely and explaining why liquid water is denser than ice. As a result, ice floats on water, and ponds and lakes freeze from the top down, and therefore do not freeze completely. This property allows fish and aquatic plants to survive winter in the cold water under the layer of ice.
Quick Check 3 Why do containers of water, milk, soda, or other liquids sometimes burst when frozen?
Quick Check 3 Answer
Ice is less dense than liquid water. As a result, when water freezes, it expands in volume and can burst closed containers, such as cans of soda or water pipes in houses. This property is unusual. For most substances, the solid phase is more dense than the liquid phase.
Hydrogen bonds also give water molecules the property of cohesion, meaning that they tend to stick to one another. A consequence of cohesion is high surface tension, a measure of the difficulty of breaking the surface of a liquid. Cohesion between molecules contributes to water movement in plants. As water evaporates from leaves, water is pulled upward, sometimes as high as 100 meters above the ground in giant sequoia and coast redwood trees, which are among the tallest trees on Earth.
37
The hydrogen bonds of water also influence how water responds to heating. Molecules are in constant motion, and this motion increases as the temperature increases. When water is heated, some of the energy added by heating is used to break hydrogen bonds instead of causing more motion among the molecules, so the temperature increases less than if there were no hydrogen bonding. The abundant hydrogen bonds make water more resistant to temperature changes than other substances, a property that is important for living organisms on a variety of scales. In the cell, water resists temperature variations that would otherwise result from numerous biochemical reactions. On a global scale, the oceans minimize temperature fluctuations, stabilizing the temperature on Earth in a range compatible with life.
In short, water is clearly the medium of life on Earth, but is this because water is uniquely suited for life, or is it because life on Earth has adapted through time to a watery environment? We don’t know the answer, but probably both explanations are partly true. Chemists have proposed that under conditions of high pressure and temperature, other small molecules, among them ammonia (NH3) and some simple carbon-