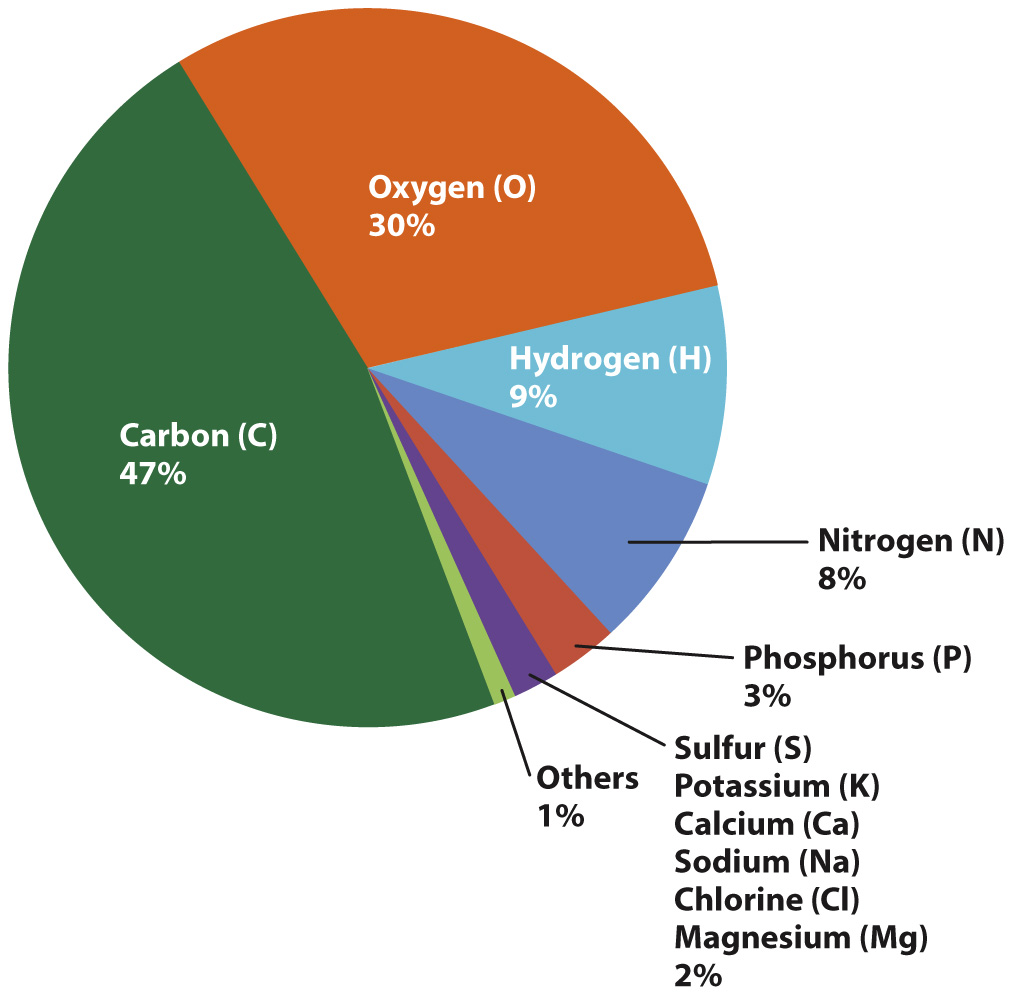
Hydrogen and helium are far and away the most abundant elements in the universe. In contrast, the solid Earth is dominated by silicon, oxygen, aluminum, iron, and calcium (Chapter 1). In other words, Earth is not a typical sample of the universe. Nor is the cell a typical sample of the solid Earth. Fig. 2.12 shows the relative abundance by mass of chemical elements present in human cells after all the water has been removed. Note that just four elements—carbon (C), oxygen (O), hydrogen (H), and nitrogen (N)—constitute 94% of the total dry mass, and that the most abundant element is carbon. The elemental composition of human cells is typical of all cells. Human life, and all life as we know it, is based on carbon. Carbon molecules play such an important role in living organisms that carbon-containing molecules have a special name—they are called organic molecules. Their central role in life implies that there must be something very special about carbon, and there is. Carbon has the ability to combine with many other elements to form a wide variety of molecules, each specialized for the functions it carries out in the cell.