Carbon-
Carbon has other properties that contribute to its ability to form a diversity of molecules. For example, carbon atoms can link with each other by covalent bonds to form long chains. These chains can be branched, or two carbons at the ends of the chain or within the chain can link to form a ring.
Among the simplest chains is ethane, shown in Fig. 2.14a. Ethane is formed when two carbon atoms become connected by a covalent bond. In this case, the orbitals of unpaired electrons in two carbon atoms form the covalent bond. Each carbon atom is also bound to three hydrogen atoms. Some more complex examples of carbon-
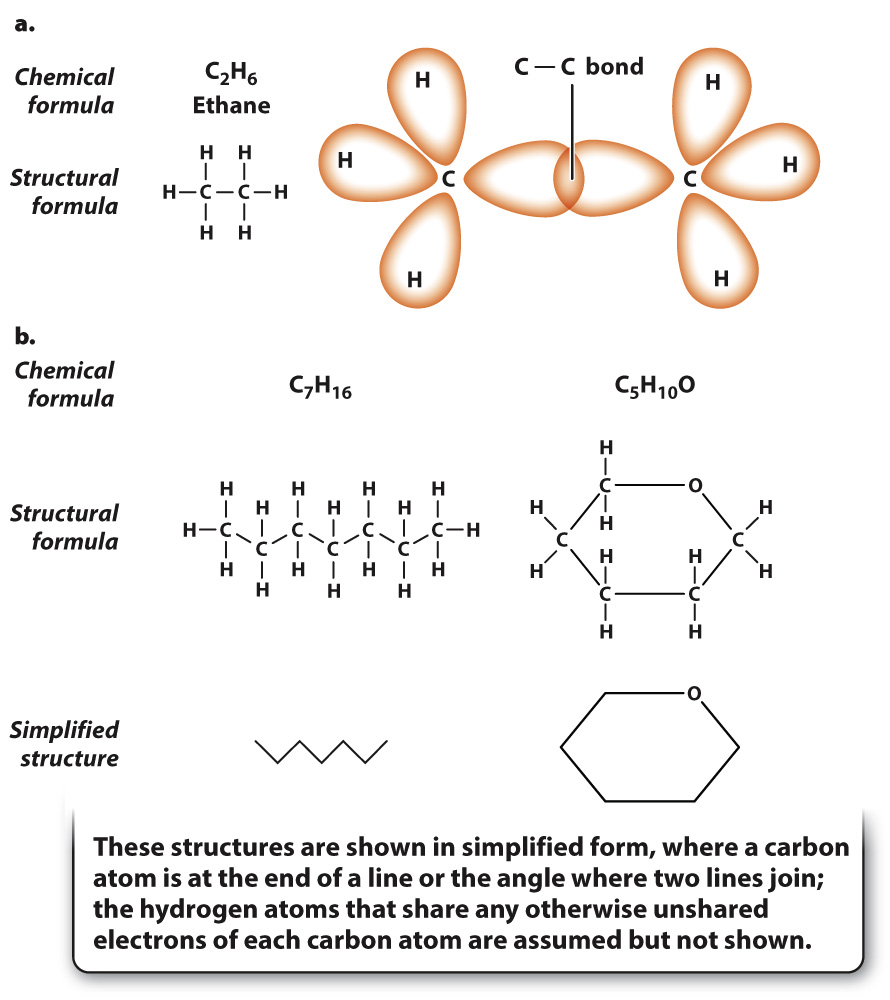
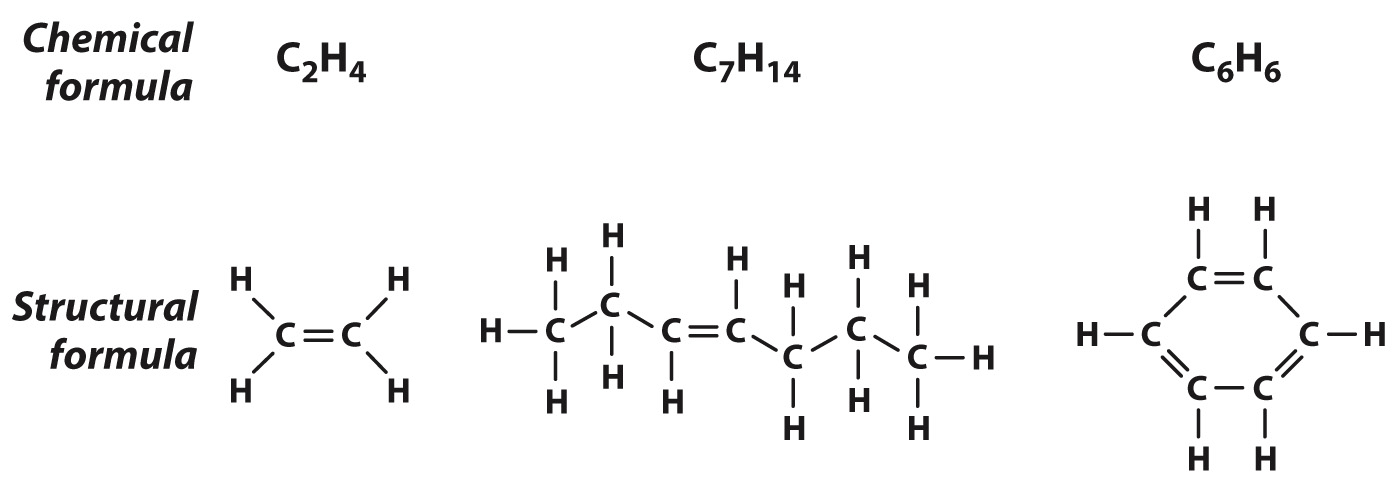
Two adjacent carbon atoms can also share two pairs of electrons, forming a double bond, as shown in Fig. 2.15. Note that each carbon atom has exactly four covalent bonds, but in this case two are shared between adjacent carbon atoms. The double bond is shorter than a single bond and is not free to rotate, so all of the covalent bonds formed by the carbon atoms connected by a double bond are in the same geometrical plane. As with single bonds, double bonds can be found in chains of atoms or ring structures.
39
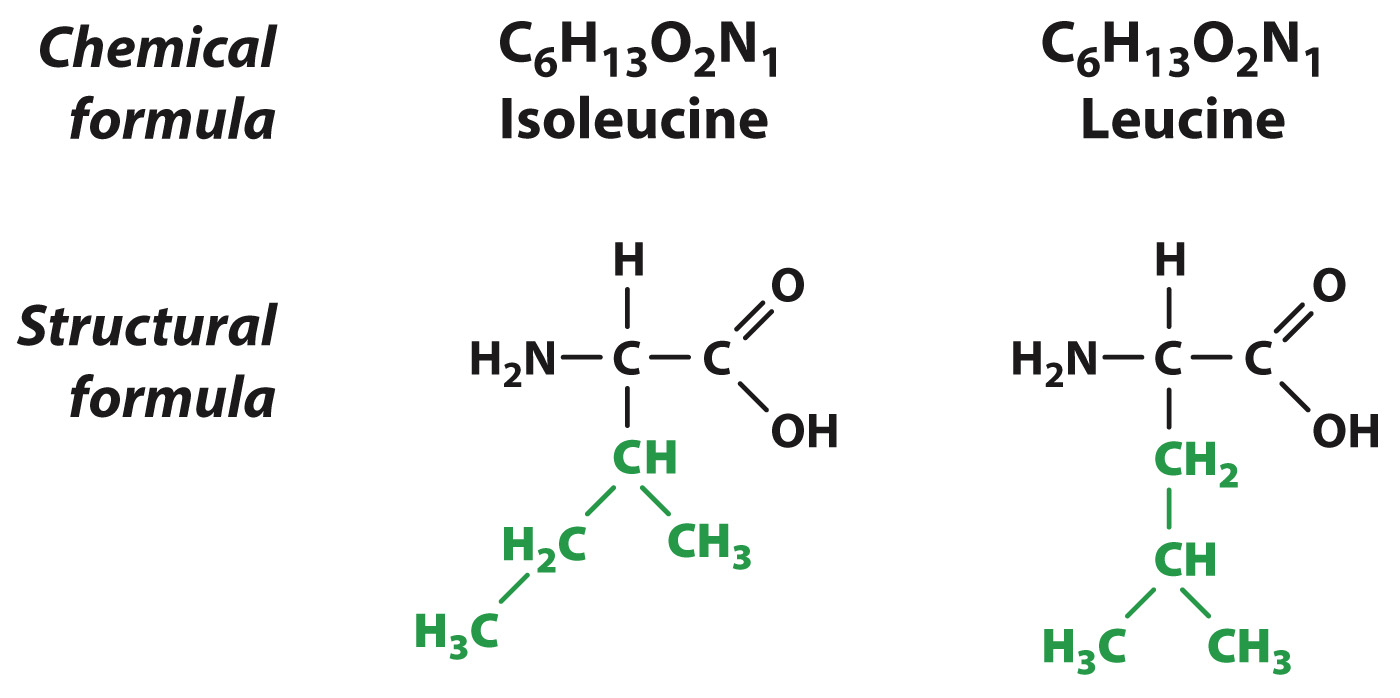
While the types of atoms making up a molecule help characterize the molecule, the spatial arrangement of atoms is also important. For example, 6 carbon atoms, 13 hydrogen atoms, 2 oxygen atoms, and 1 nitrogen atom can join covalently in many different arrangements to produce molecules with different structures. Two of these many arrangements are shown in Fig. 2.16. Note that some of the connections between atoms are identical in the two molecules (black) and some are different (green), even though the chemical formulas are the same (C6H13O2N1). Molecules that have the same chemical formula but different structures are known as isomers.
We have seen that carbon-