Proteins are composed of amino acids.
Proteins do much of the cell’s work. Some function as catalysts that accelerate the rates of chemical reactions (in which case they are called enzymes), and some act as structural components necessary for cell shape and movement. Your body contains many thousands of distinct types of protein that perform a wide range of functions. Since proteins consist of amino acids linked covalently to form a chain, we need to examine the chemical features of amino acids to understand the diversity and versatility of proteins.
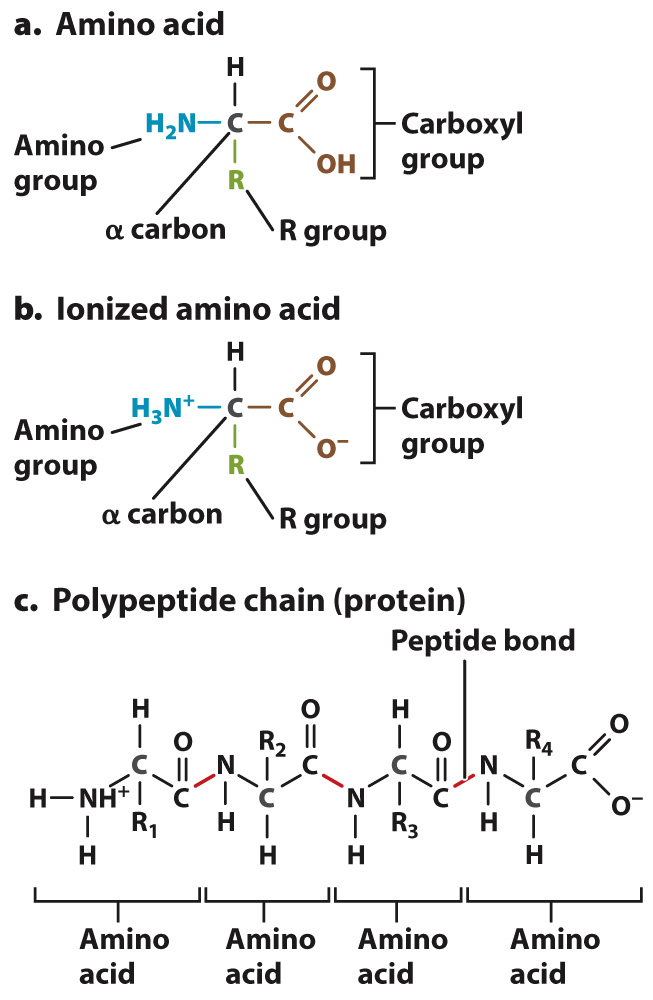
The general structure of an amino acid is shown in Fig. 2.17a. Each amino acid contains a central carbon atom, called the α (alpha) carbon, covalently linked to four groups: an amino group (–NH2; blue), a carboxyl group (–COOH; brown), a hydrogen atom (H), and an R group, or side chain, (green) that differs from one amino acid to the next. The identity of each amino acid is determined by the structure and composition of the side chain. The side chain of the amino acid glycine is simply H, for example, and that of alanine is CH3. In most amino acids, the α carbon is covalently linked to four different groups. Glycine is the exception, since its R group is a hydrogen atom.
At the pH commonly found in a cell (pH 7.4), the amino and carboxyl groups are ionized (charged), with the amino group gaining a proton ( ; blue) and the carboxyl group losing a proton (–COO–; brown), as shown in Fig. 2.17b.
Amino acids are linked in a chain to form a protein (Fig. 2.17c). The carbon atom in the carboxyl group of one amino acid is joined to the nitrogen atom in the amino group of the next by a covalent linkage called a peptide bond. In Fig. 2.17c, the chain of amino acids includes four amino acids, and the peptide bonds are indicated in red. The formation of a peptide bond involves the loss of a water molecule since in order to form a C–
Cellular proteins are composed of combinations of 20 different amino acids, each of which can be classified according to the chemical properties of its R group. The particular sequence, or order, in which amino acids are present in a protein determines how it folds into its three-