The building blocks of life can be generated in the laboratory.
Research into the origins of life was catapulted into the experimental age in 1953 with an elegant experiment carried out by Stanley Miller, then a graduate student in the laboratory of Nobel laureate Harold Urey. Miller started with gases such as water vapor, methane, and hydrogen gas, all thought to have been present in the early atmosphere. He put these gases into a sealed flask and then passed a spark through the mixture (Fig. 2.28). On the primitive Earth, lightning might have supplied the energy needed to drive chemical reactions, and the spark was meant to simulate its effects. Analysis of the contents of the flask showed that a number of amino acids were generated.
46
HOW DO WE KNOW?
FIG. 2.28
Could the building blocks of organic molecules have been generated on the early Earth?
BACKGROUND In the 1950s, Earth’s early atmosphere was widely believed to have been rich in water vapor, methane, ammonia, and hydrogen gas, with no free oxygen.
EXPERIMENT Stanley Miller built an apparatus, shown below, designed to simulate Earth’s early atmosphere. Then he passed a spark through the mixture to simulate lightning.
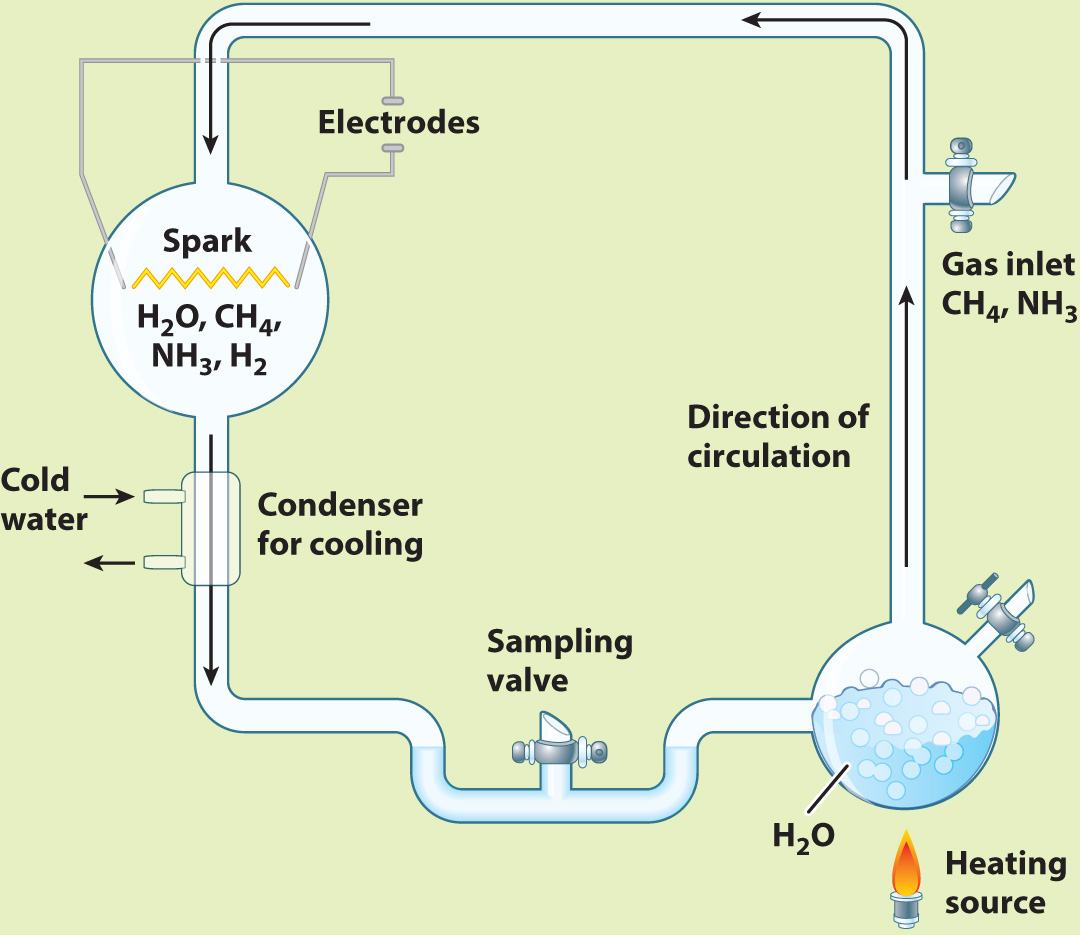
RESULTS As the experiment proceeded, reddish material accumulated on the walls of the flask. Analysis showed that the brown matter included a number of amino acids.
CONCLUSION Amino acids can be generated in conditions that mimic those of the early Earth.
FOLLOW-
SOURCES Miller, S. L. 1953. “Production of Amino Acids Under Possible Primitive Earth Conditions.” Science 117:528–
Miller and others conducted many variations on his original experiment, all with similar results. Today, many scientists doubt that the early atmosphere had the composition found in Miller’s experimental apparatus, but amino acids and other biologically important molecules can form in a variety of simulated atmospheric compositions. If oxygen gas (O2) is absent and hydrogen is more common in the mixture than carbon, the addition of energy generates diverse amino acids. The absence of oxygen gas is critical since these types of reactions cannot run to completion in modern air or seawater. Here, however, geology supports the experiments: Chemical analyses of Earth’s oldest sedimentary rocks indicate that, for its first 2 billion years, Earth’s surface contained little or no oxygen.
Later experiments have shown that other chemical reactions can generate simple sugars, the bases found in nucleotides, and the lipids needed to form primitive membranes. Independent evidence that simple chemistry can form the building blocks of life comes from certain meteorites, which provide samples of the early solar system and contain diverse amino acids, lipids, and other organic molecules.