Chapter 2 Summary
Core Concepts Summary
2.1 The atom is the fundamental unit of matter.
Atoms consist of positively charged protons and electrically neutral neutrons in the nucleus, as well as negatively charged electrons moving around the nucleus. page 30
The number of protons determines the identity of an atom. page 30
The number of protons and neutrons together determines the mass of an atom. page 30
The number of protons versus the number of electrons determines the charge of an atom. page 30
Negatively charged electrons travel around the nucleus in regions called orbitals. page 30
The periodic table of the elements reflects a regular and repeating pattern in the chemical behavior of elements. page 31
2.2 Atoms can combine to form molecules linked by chemical bonds.
Valence electrons occupy the outermost energy level (shell) of an atom and determine the ability of an atom to combine with other atoms to form molecules. page 33
A covalent bond results from the sharing of electrons between atoms to form a molecular orbital. page 33
A polar covalent bond results when two atoms do not share electrons equally as a result of a difference in the ability of the atoms to attract electrons, a property called electronegativity. page 33
An ionic bond results from the attraction of oppositely charged ions. page 34
2.3 Water is abundant and essential for life.
Water is a polar molecule because shared electrons are distributed asymmetrically between the oxygen and hydrogen atoms. page 35
Hydrophilic molecules dissolve readily in water, whereas hydrophobic molecules in water tend to associate with one another, minimizing their contact with water. page 35
A hydrogen bond results when a hydrogen atom covalently bonded to an electronegative atom interacts with an electronegative atom of another molecule. page 35
Water forms hydrogen bonds, which help explain its high cohesion, surface tension, and resistance to rapid temperature change. page 36
48
The pH of an aqueous solution is a measure of the acidity of the solution. page 37
2.4 Carbon is the backbone of organic molecules.
A carbon atom can form up to four covalent bonds with other atoms. page 38
The geometry of these covalent bonds helps explain the structural and functional diversity of organic molecules. page 38
2.5 Organic molecules include proteins, nucleic acids, carbohydrates, and lipids, each of which is built from simpler units.
Amino acids are linked by covalent bonds to form proteins. page 40
An amino acid consists of a carbon atom (the α carbon) attached to a carboxyl group, an amino group, a hydrogen atom, and a side chain. page 40
The side chain determines the properties of an amino acid. page 40
Nucleotides assemble to form nucleic acids, which store and transmit genetic information. page 40
Nucleotides are composed of a 5-
Nucleotides in DNA incorporate the sugar deoxyribose, and nucleotides in RNA incorporate the sugar ribose. page 41
The bases are pyrimidines (cytosine, thymine, and uracil) and purines (guanine and adenine). page 41
Sugars are carbohydrates, molecules composed of C, H, and O atoms, usually in the ratio 1:2:1, and are a source of energy. page 42
Monosaccharides assemble to form disaccharides or longer polymers called complex carbohydrates. page 42
Lipids are hydrophobic. page 43
Triacylglycerols store energy and are made up of glycerol and fatty acids. page 43
Fatty acids consist of a linear hydrocarbon chain of variable length with a carboxyl group at one end. page 43
Fatty acids are either saturated (no carbon–
The tight packing of fatty acids in lipids is the result of van der Waals forces, a type of weak, noncovalent bond. page 44
2.6 Life likely originated on Earth by a set of chemical reactions that gave rise to the molecules of life.
In 1953, Stanley Miller and Harold Urey demonstrated that amino acids can be generated in the laboratory in conditions that mimic those of the early Earth. page 45
Other experiments have shown that sugars, bases, and lipids can be generated in the laboratory. page 46
Once the building blocks were synthesized, they could join together in the presence of clay minerals to form polymers. page 46
Self-Assessment
Name and describe the components of an atom.
Self-Assessment 1 Answer
An atom is made up of positively charged particles called protons, neutral particles called neutrons, and negatively charged particles called electrons. The dense central nucleus of an atom is made up of protons and neutrons. Electrons orbit around the nucleus, and the regions of space where they are most likely to be found are called orbitals.
Explain how the periodic table of the elements is organized.
Self-Assessment 2 Answer
The periodic table of elements is organized by the increasing atomic number of each atom. The atomic number is the number of protons an atom has in its nucleus. Hydrogen appears first in the periodic table because it has an atomic number of 1. The elements in a column share similar chemical properties, and each has the same number of electrons in its outermost orbital. Elements in a row have the same number of shells (energy levels), and thus the same number and types of orbitals.
Differentiate between covalent bonds and generally weaker interactions such as polar covalent, hydrogen, and ionic bonds.
Self-Assessment 3 Answer
A covalent bond is present when two atoms share their valence electrons (the electrons in the outermost orbital of an atom). Each shared pair of valence electrons make a covalent bond that is depicted by a single line connecting the two chemical symbols for the atoms. A polar covalent bond is present when the valence electrons are not shared equally by the two atoms, thus giving areas of the molecule a positive or negative charge. A hydrogen bond forms when a hydrogen atom covalently bound to an electronegative atom (giving the hydrogen a partial positive charge) interacts with an electronegative atom of another molecule. A hydrogen bond is typically depicted by a dotted line. An ionic bond is formed by the attraction between a molecule that has a positive charge (due to the loss of one electron) and a molecule that has a negative charge (due to the gain of one electron). The two molecules are not covalently bound, but they associate with each other due to their opposite charges.
List three unusual properties of water and explain why these properties make water conducive to life.
Self-Assessment 4 Answer
Water has unusual properties and is conducive to life in the following ways: Water is a polar molecule, and because of its regions of positive and negative charge, some molecules are attracted to water (hydrophilic) and some are repelled by it (hydrophobic). It is this property that allows things like lipid cellular membranes, and thus cells, to exist. The polar nature of water also makes it a good solvent—
hydrophilic compounds dissolve readily in water. Water also has a neutral pH (around 7), the pH of most cells. Since many chemical reactions can only be carried out in a solution around a neutral pH, it is important that the cell remain in this range to function. Water resists temperature changes better than other substances due to its extensive network of hydrogen bonding. This is important for a variety of reasons. This phenomenon allows chemical reactions, which produce heat as a by- product, to occur inside the cell without changing the internal temperature. In a similar way, but on a global scale, the oceans act as a temperature regulator and keep the Earth in a temperature range that supports life. The network of hydrogen bonds that forms when water freezes makes ice less dense than liquid water. As a result, ice floats on water, which allows aquatic life to survive below the ice in the winter. Finally, the cohesive properties and surface tension of water facilitate water transportation in plants. List the four most common elements in organic molecules and state which common macromolecules always contain all four of these elements.
Self-Assessment 5 Answer
The four most common elements in organic molecules in order of decreasing abundance by dry mass are carbon, oxygen, hydrogen, and nitrogen. All four of these elements are found in proteins and nucleic acids because they are parts of the building blocks of these polymers.
List features of carbon that allow it to form diverse structures.
Self-Assessment 6 Answer
Features of carbon that allow it to form diverse structures are the following: A carbon atom behaves as if it has four unpaired electrons, allowing it to form covalent bonds with up to four different atoms. Each of these bonds can also rotate freely, contributing to the structural diversity of carbon-
based molecules. Carbon atoms can bond with other carbon atoms to form large carbon chains that branch or form rings, also giving rise to a great diversity of structures. Carbon can also form double bonds (sharing two electrons). A double bond does not freely rotate, which limits the flexibility of the molecule and its structural options. List essential functions of proteins, nucleic acids, carbohydrates, and lipids.
Self-Assessment 7 Answer
Proteins act as catalysts to facilitate chemical reactions and also provide structural support of the cell. Nucleic acids encode and transmit genetic information. Carbohydrates provide a source of energy and make up the cell wall in bacteria, plant, and algae cells. Lipids store energy, act as signaling molecules, and make up the membranes of the cell.
Describe how diversity is achieved in polymers, using proteins as an example.
Self-Assessment 8 Answer
Diversity is achieved in polymers through endless combinations of subunits. A protein, for example, is a polymer of amino acid subunits. As we will discuss in Chapter 4, there are 20 different kinds of amino acids. Thus, there are numerous combinations of subunits that could be made, each resulting in a different protein. In this way, polymers are capable of displaying virtually limitless diversity.
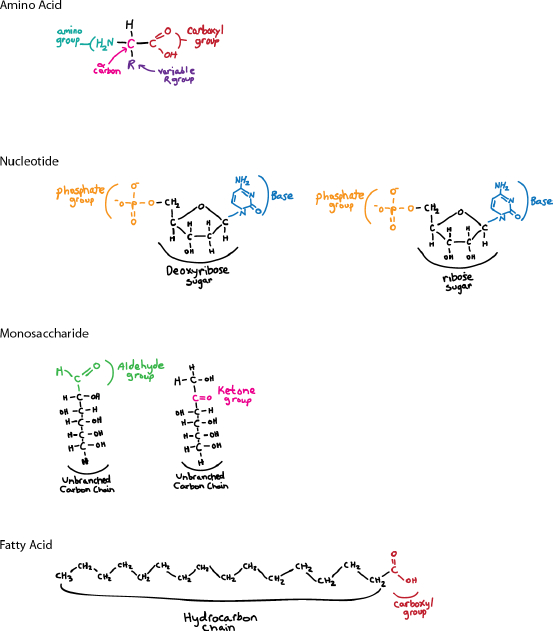
Sketch the basic structures of amino acids, nucleotides, monosaccharides, and fatty acids.
Self-Assessment 9 Answer
What evidence is there for the hypothesis that life originated on Earth by the creation and polymerization of small organic molecules by natural processes?
Self-Assessment 10 Answer
The principal molecules found in organisms are themselves made of simpler molecules joined together. So if we want to understand how proteins emerged on Earth, then we first have to understand the synthesis of amino acids. Stanley Miller conducted experiments that showed that when a mixture of gases―which were thought to have been present in the early atmosphere―were ignited with a spark, amino acids were generated. Other scientists have subsequently shown that the other building blocks of life—
sugars, bases, and lipids— can also be formed in laboratory conditions that simulate the early atmosphere. Analyses of meteorites that provide samples of the early solar system have also shown the presence of diverse amino acids, lipids, and other organic compounds, which supports Miller’s initial hypothesis. Building off of Miller’s work, Leslie Orgel performed experiments that showed how nucleotides would spontaneously join to synthesize nucleic acids. Many years later, John Sutherland and his colleagues were able to synthesize nucleotides themselves under conditions thought to be like those on the early Earth.