Electrons occupy regions of space called orbitals.
Electrons move around the nucleus, but not in the simplified way shown in Fig. 2.1. The exact path that an electron takes cannot be known, but it is possible to identify a region in space, called an orbital, where an electron is present most of the time. For example, Fig. 2.2a shows the orbital for hydrogen, which is simply a sphere occupied by a single electron. Most of the time, the electron is found within the space defined by the sphere, although its exact location at any instant is unpredictable.
31
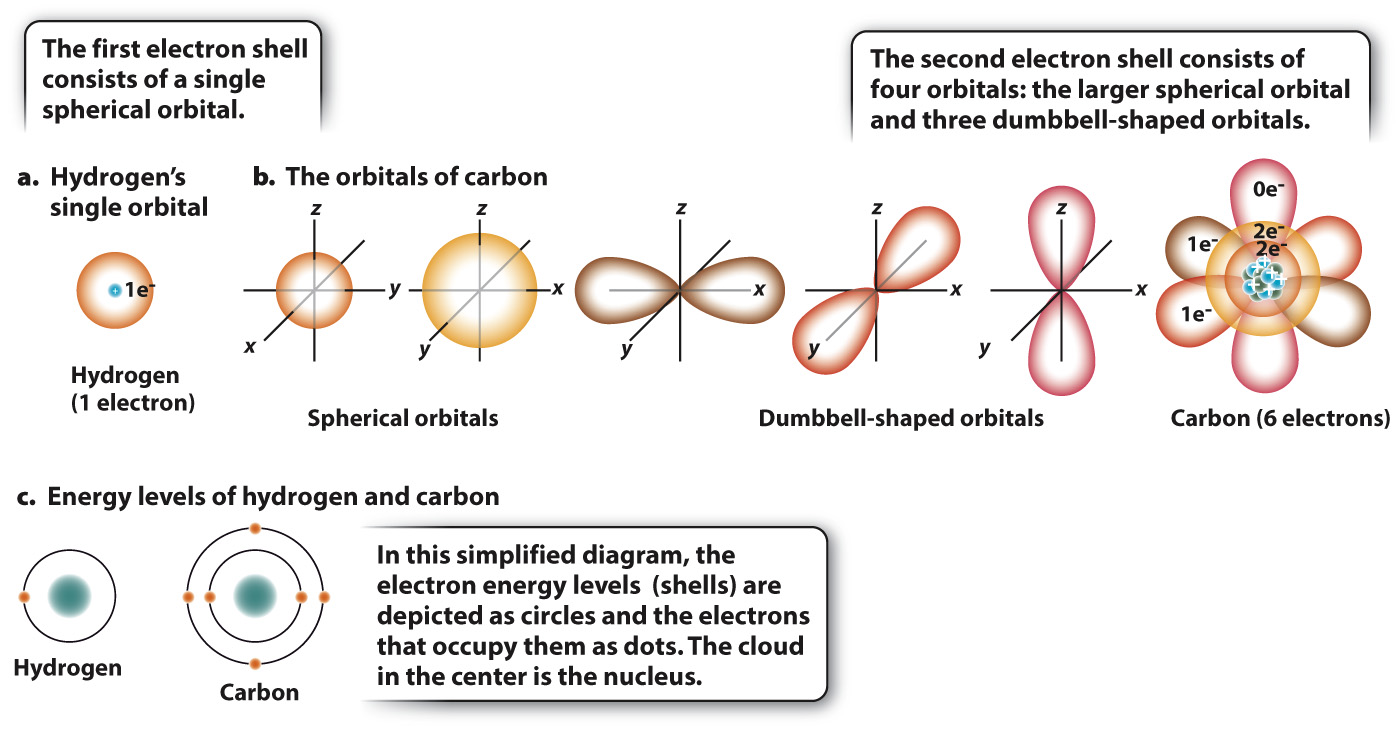
The maximum number of electrons in any orbital is two. Most atoms have more than two electrons and so have several orbitals positioned at different distances from the nucleus. These orbitals differ in size and shape. Electrons in orbitals close to the nucleus have less energy than do electrons in orbitals farther away, so electrons fill up orbitals close to the nucleus before occupying those farther away. Several orbitals can exist at a given energy level, or shell. The first shell consists of the spherical orbital shown in Fig. 2.2a.
Fig. 2.2b shows electron orbitals for carbon. Of carbon’s six electrons, two occupy the small spherical orbital representing the lowest energy level. The remaining four are distributed among four possible orbitals at the next highest energy level: One of these four orbitals is a sphere (larger in diameter than the orbital at the lowest energy level) and three are dumbbell-
Quick Check 1 In the early 1900s, Ernest Rutherford produced a beam of very small positive particles and directed it at a thin piece of gold foil just a few atoms thick. Most of the particles passed through the foil without changing their path; very rarely, a particle was deflected. What conclusions can you draw from this experiment about the structure of an atom?
Quick Check 1 Answer
One conclusion is that atoms consist mainly of empty space, and hence most positively charged particles passing through the gold foil do not come close enough to any other positive charge to be deflected. Another conclusion is that the positively charged protons in the nucleus must be small and densely packed.