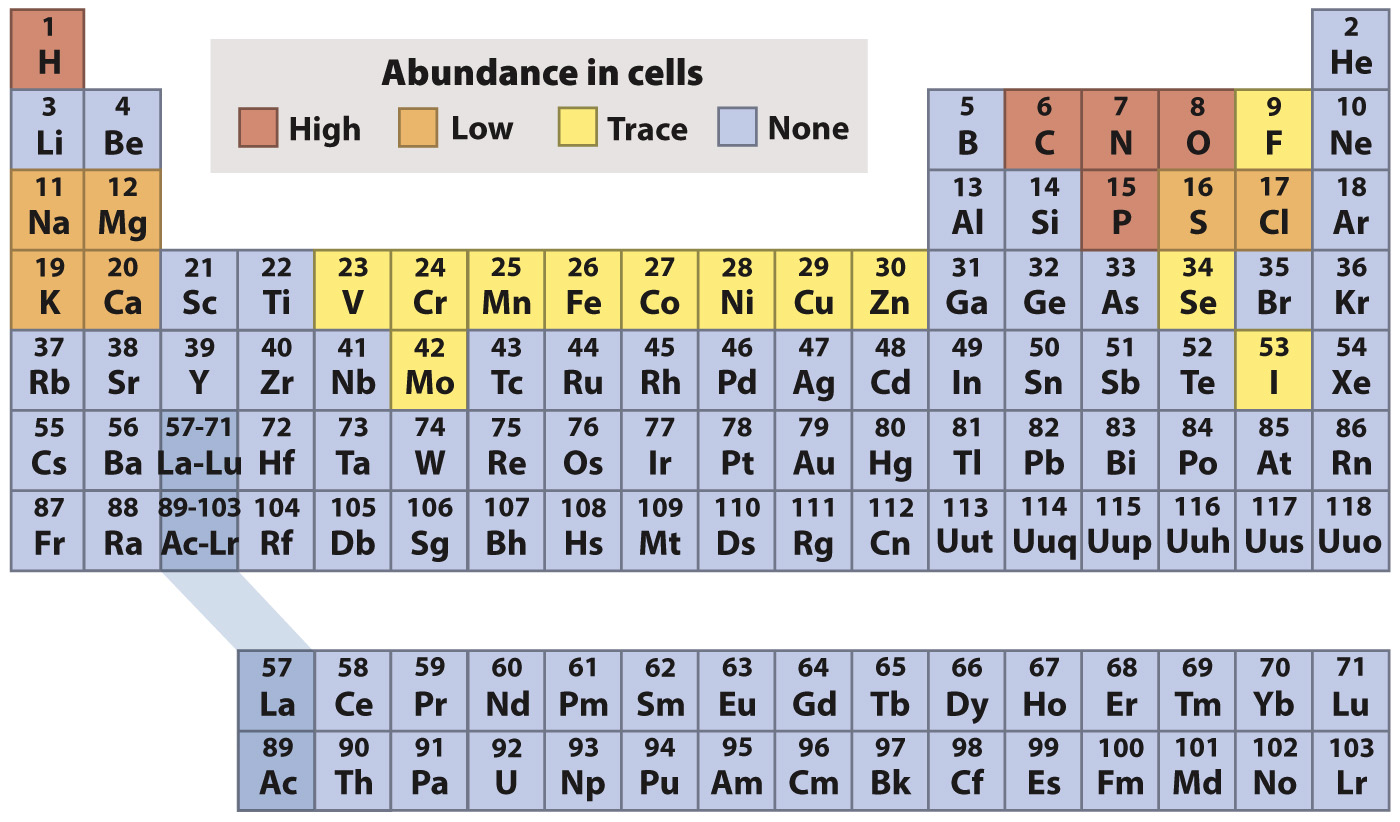
Elements have recurring, or periodic, chemical properties.
The chemical elements are often arranged in a tabular form known as the periodic table of the elements, shown in Fig. 2.3 and generally credited to the nineteenth-
32
In the periodic table, the elements are indicated by their chemical symbols and arranged in order of increasing atomic number. For example, the second row of the periodic table begins with lithium (Li) with 3 protons and ends with neon (Ne) with 10 protons.
For the first three horizontal rows in the periodic table, elements in the same row have the same number of shells, and so also have the same number and types of orbitals available to be filled by electrons. Across a row, therefore, electrons fill the shell until a full complement of electrons is reached on the right-
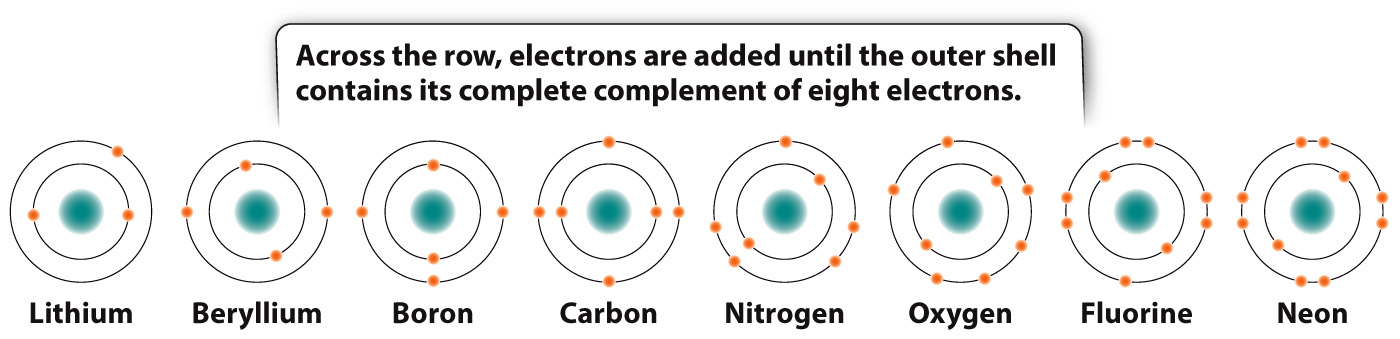
The elements in a vertical column are called a group or family. Members of a group all have the same number of electrons in their outermost shell. For example, carbon (C) and lead (Pb) both have four electrons in their outermost shell. The number of electrons in the outermost shell determines in large part how elements interact with other elements to form a diversity of molecules, as we will see in the next section.