A covalent bond results when two atoms share electrons.
33
The ability of atoms to combine with other atoms is determined in large part by the electrons farthest from the nucleus—
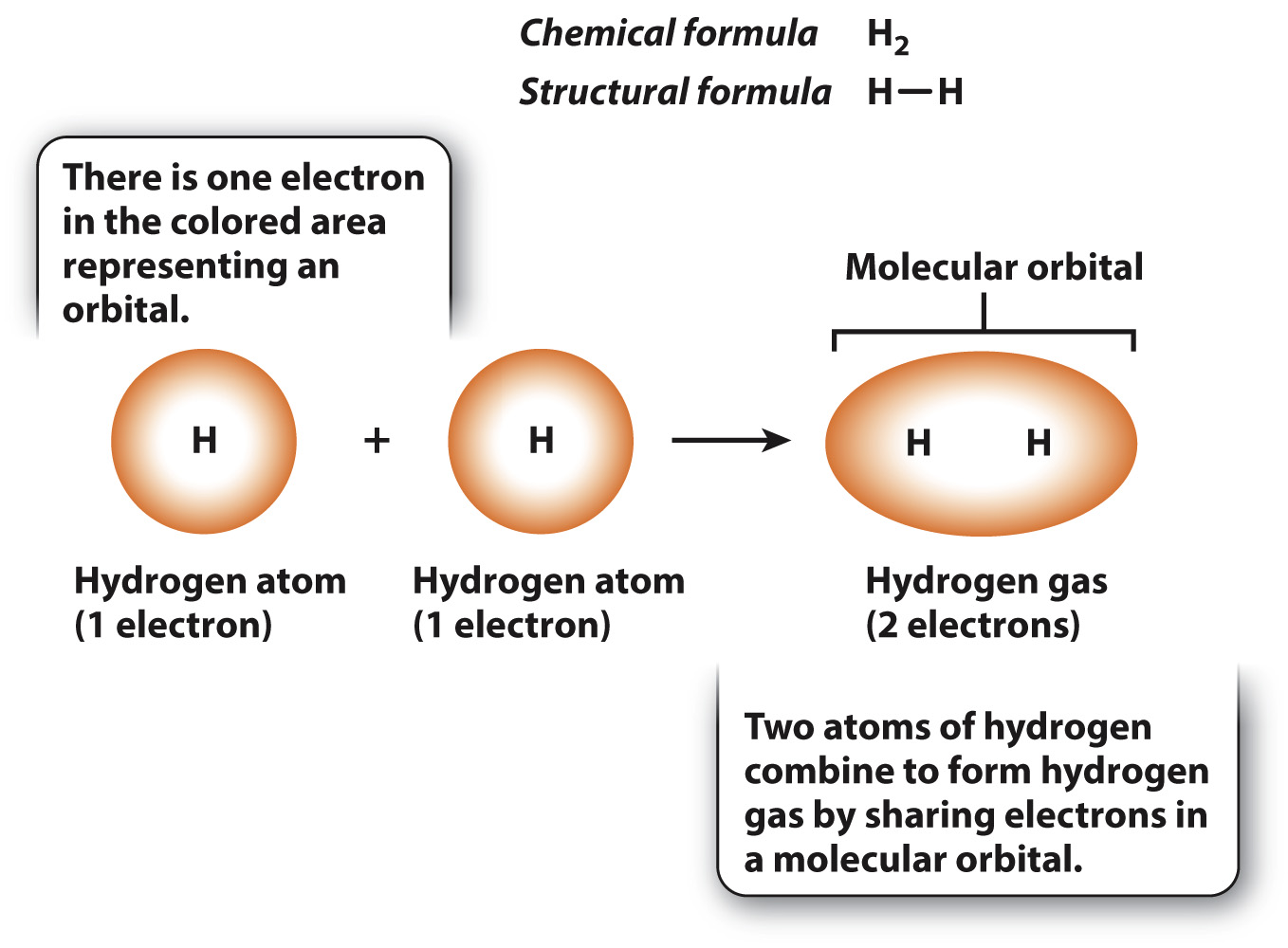
We can represent a specific molecule by its chemical formula, which is written as the letter abbreviation for each element followed by a subscript giving the number of that type of atom in the molecule. Among the simplest molecules is hydrogen gas, illustrated in Fig. 2.5, which consists of two covalently bound hydrogen atoms indicated by the chemical formula H2. Each hydrogen atom has a single electron in a spherical orbital. When the atoms join into a molecule, the two orbitals merge into a single molecular orbital containing two electrons that are shared by the hydrogen atoms. A covalent bond between atoms is denoted by a single line connecting the two chemical symbols for the atoms, as shown in the structural formula in Fig. 2.5.
Quick Check 2 From their positions in the periodic table (see Fig. 2.3), can you predict how many lithium atoms and hydrogen atoms can combine to form a molecule?
Quick Check 2 Answer
Hydrogen and lithium are in the same column, or group, in the periodic table. Each has one valence electron in their outer orbital. As a result, one atom of lithium combines with one atom of hydrogen to make lithium hydride, with a full complement of two electrons in the single molecular orbital.
Two adjacent atoms can sometimes share two pairs of electrons, forming a double bond denoted by a double line connecting the two chemical symbols for the atoms. In this case, four orbitals occupied by a single electron merge to form two molecular orbitals.
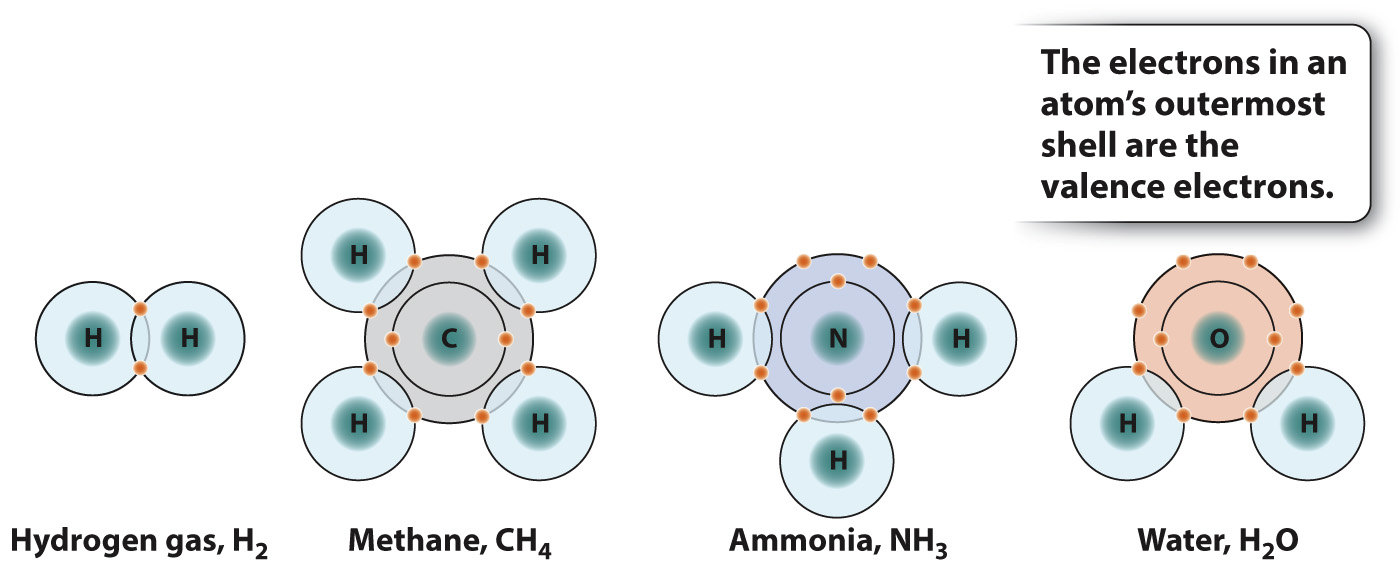
Molecules tend to be most stable when the two atoms forming a bond share enough electrons to completely occupy the outermost energy level or shell. The outermost shell of a hydrogen atom can hold two electrons, whereas for carbon, nitrogen, and oxygen atoms the number is eight. This simple rule for forming stable molecules is known as the octet rule and applies to many, but not all, elements. For example, as shown in Fig. 2.6, one carbon atom (C, with four valence electrons) combines with four hydrogen atoms (H, with one valence electron each) to form CH4 (methane); nitrogen (N, with five valence electrons) combines with three H atoms to form NH3 (ammonia); and oxygen (O, with six valence electrons) combines with two H atoms to form H2O (water). Interestingly, the elements of the same column in the next row behave similarly. This is just one example of the recurring, or periodic, behavior of the elements.