A polar covalent bond is characterized by unequal sharing of electrons.
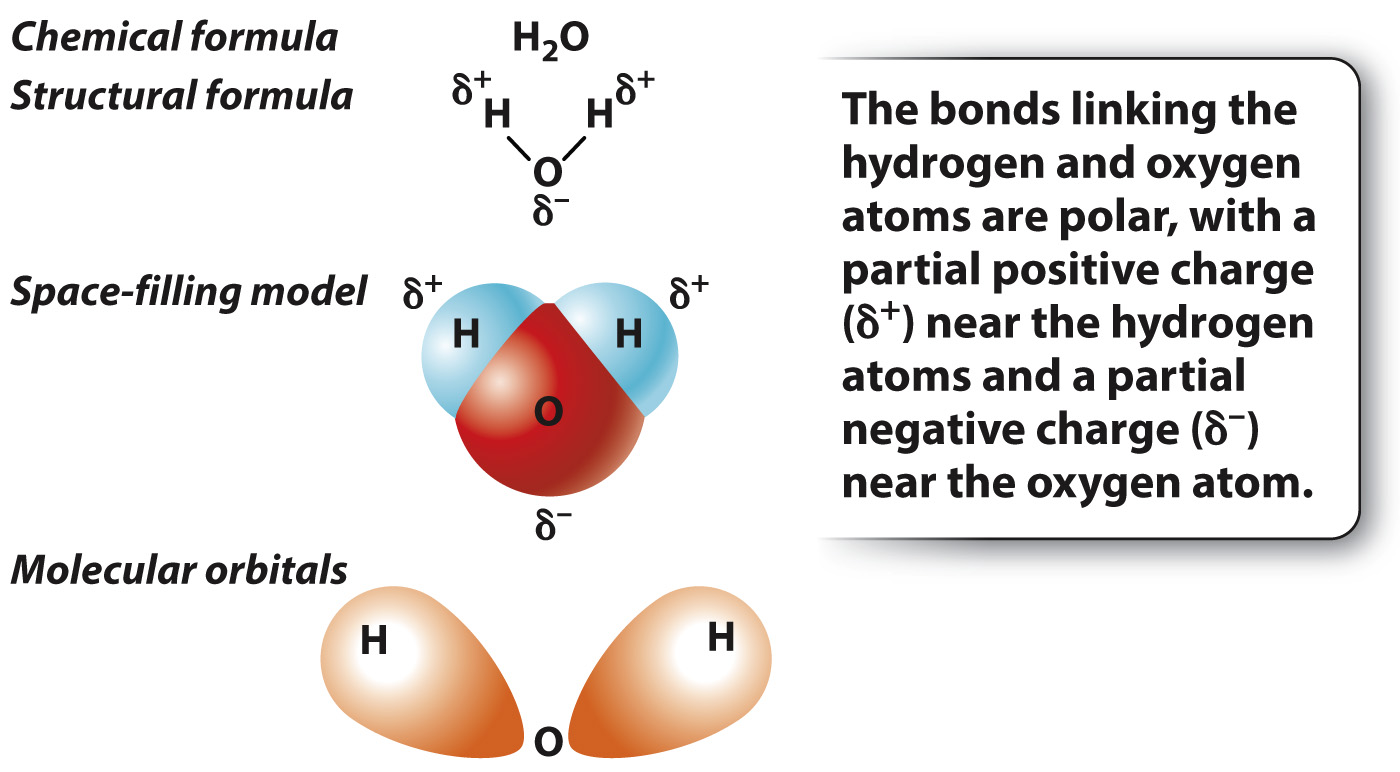
In hydrogen gas (H2), the electrons are shared equally by the two hydrogen atoms. In many bonds, however, the electrons are not shared equally by the two atoms. A notable example is provided by the bonds in a water molecule (H2O), which consists of two hydrogen atoms each covalently bound to a single oxygen atom (Fig. 2.7).
34
In a molecule of water, the electrons are more likely to be located near the oxygen atom. The unequal sharing of electrons results from a difference in the ability of the atoms to attract electrons, a property known as electronegativity. Electronegativity tends to increase across a row in the periodic table; as the number of protons across a row increases, electrons are held more tightly to the nucleus. Therefore, oxygen is more electronegative than hydrogen and attracts electrons more readily than does hydrogen. In a molecule of water, oxygen has a slight negative charge, while the two hydrogen atoms have a slight positive charge (Fig. 2.7). When electrons are shared unequally between the two atoms, the resulting interaction is described as a polar covalent bond.
A covalent bond between atoms that have the same, or nearly the same, electronegativity is a nonpolar covalent bond, which means that the atoms share the bonding electron pair almost equally. Nonpolar covalent bonds include those in gaseous hydrogen (H2) and oxygen (O2), as well as carbon–