Neurons are excitable cells that transmit information by action potentials.
When a nerve cell is excited, its membrane potential becomes less negative—that is, the inside becomes less negative, or more positive, than the outside of the cell. The increase in membrane potential is therefore referred to as a depolarization of the membrane. This depolarization does not occur over the entire cell at once. Rather, it starts at the terminal end of the dendrite, in response to neurotransmitter binding to membrane receptors, and travels to the cell body, losing strength along the way. If the depolarization is still strong enough at the axon hillock of the cell body, the cell fires an action potential that carries the signal from the cell body to the terminal ends of the axon.
How is the action potential generated? At the axon hillock, the summed membrane depolarization of the cell’s dendrites causes voltage-gated sodium channels to open, allowing Na+ ions to enter the cell. Voltage-gated channels open and close in response to changes in membrane potential (Fig. 35.8; Chapter 9). As Fig. 35.8 shows, they play a key role in how neurons fire action potentials.
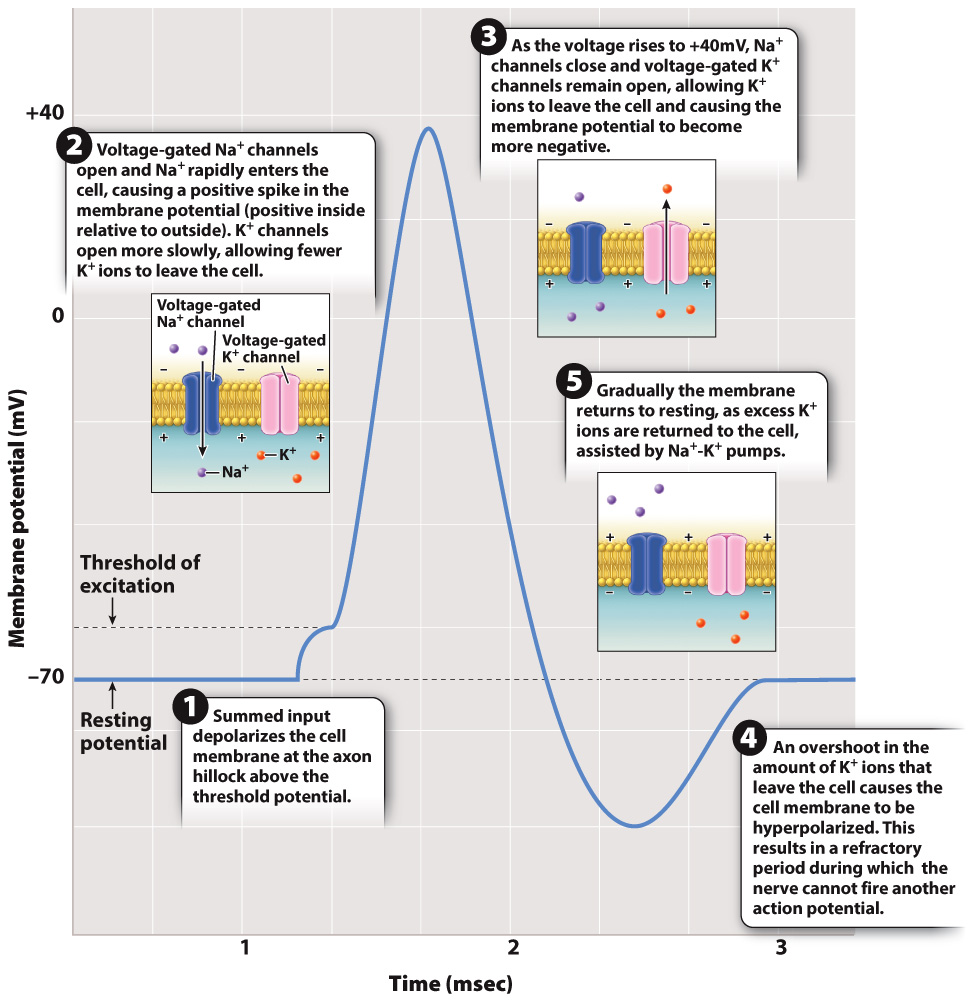
FIG. 35.8 An action potential. An action potential results from the opening and closing of voltage-gated ion channels and allows the membrane potential briefly to be positively charged.
The influx of Na+ causes a flow of positive charge, like a current, along the inside of the cell, toward more negatively charged nearby regions. This flow of charge is reduced at longer distances and quickly dissipates so that the membrane potential returns to a resting state unless additional excitatory stimuli further depolarize the membrane.
If the excitatory signal is strong enough to depolarize the membrane of the nerve cell body to a voltage of approximately 15 mV above the resting membrane potential (about –55 mV), the nerve fires an action potential at the axon hillock. An action potential is a rapid, short-lasting rise and fall in membrane potential, as shown in Fig. 35.8. The critical depolarization voltage of –55 mV required for an action potential is the cell’s threshold potential. Nerve cells in different animals have slightly different resting and threshold potentials, but all operate similarly with respect to firing an action potential. When the threshold potential is exceeded, the nerve fires an action potential in an all-or-nothing fashion. This means that once the threshold potential has been exceeded, the magnitude of the action potential is always the same and is independent of the strength of the stimulating input. At this point, a rapid spike in voltage to approximately +40 mV occurs over a very brief time period of 1 to 2 msec.
An action potential results from dramatic changes in the state of voltage-gated Na+ and voltage-gated K+ channels in the axon membrane. As the cell membrane crosses its threshold potential, a large number of voltage-gated Na+ channels suddenly open, allowing Na+ ions to rush inside the cell. The rise in voltage that results causes additional voltage-gated Na+ channels to open. This is an example of positive feedback, in which a signal (depolarization) causes a response (open voltage-gated Na+ channels) that leads to an enhancement of the signal (more depolarization) that leads to an even larger response (more open voltage-gated Na+ channels).
During the rising phase of the action potential, voltage-gated K+ channels also begin to open, but these channels are slow to activate relative to the rapidly opening voltage-gated Na+ channels. As a result, relatively few K+ ions leave the cell and the cell membrane potential continues to rise due to the large number of Na+ ions entering the cell.
As you can see from Fig. 35.8, the rising phase of the action potential (rapid depolarization) is followed by the falling phase (rapid repolarization). What causes this sudden reversal of the membrane potential? There are two important factors. First, voltage-gated Na+ channels begin to close once the membrane potential becomes positive. Second, voltage-gated K+ channels continue to open in response to the change in voltage. Because they are slower to respond than the voltage-gated Na+ channels, the membrane voltage peaks and then falls as K+ ions diffuse out of the axon through the open voltage-gated K+ channels.
The voltage inside the axon does not return immediately to the resting membrane potential. Instead, it briefly falls below the resting potential (in what is known as hyperpolarization or undershoot), then returns to the resting potential after another few milliseconds as K+ channels close to restore the resting concentration of Na+ and K+ ions on either side of the cell membrane. The continuous action of the sodium-potassium pumps also helps to reestablish the resting membrane potential.
The period during which the inner membrane voltage falls below and then returns to the resting potential is the refractory period (Fig. 35.8). During the refractory period, a neuron cannot fire a second action potential. The refractory period results in part from the fact that when voltage-gated Na+ channels close, they require a certain amount of time before they will open again in response to a new wave of depolarization. In addition, open voltage-gated K+ channels make it difficult for the cell to reach the threshold potential. The duration of the refractory period varies for different kinds of nerve cell but limits a nerve cell’s fastest firing frequency to less than 200 times per second. Most nerve cells fire at lower rates.
It might seem that many Na+ and K+ ions need to diffuse across the axon membrane to produce an action potential. However, only about 1 in 10 million Na+ and K+ ions need to cross the membrane. Thus, enough ions are always available to generate repeated action potentials.
The nature of the action potential, which is both all-or-nothing and uniform in its electrical features, means that the action potential itself contains little information. Most neurons code information by changing the rate and timing of action potentials. Generally, a higher firing frequency codes for a more intense stimulus, such as a brighter light or louder noise, or a stronger signal transmitted by the nerve cell to other cells it contacts.
