Blood is composed of fluid and several types of cell.
In addition to its role in oxygen and carbon dioxide transport, which is our focus in this chapter, blood serves many other functions. These include heat transport for temperature regulation and nutrient transport to metabolically active tissues (Chapter 40), hormone transport (Chapter 38), waste transport to excretory organs (Chapter 41), and transport of immune cells involved in fighting pathogens (Chapter 43), as well as wound healing. Blood can be separated into a cellular fraction and a fluid fraction, or blood plasma. Red blood cells that carry hemoglobin constitute most of the cellular fraction of blood. In both vertebrates and invertebrates, hemoglobin evolved as a specialized iron-
836
The fraction of red blood cells within the blood of vertebrates is defined as the hematocrit. Whereas a hematocrit of 45% is considered normal for humans (Fig. 39.11), fish have hematocrits that range from 20% to 35%, crocodiles have hematocrits ~20%, birds have hematocrits that vary from 35% to 58%, and diving mammals have hematocrits as high as 65%. Blood with a lower hematocrit flows with less resistance (that is, has a lower viscosity), but carries less oxygen. Humans with low hematocrits are considered to be iron deficient because their blood has fewer red blood cells and therefore too little hemoglobin.
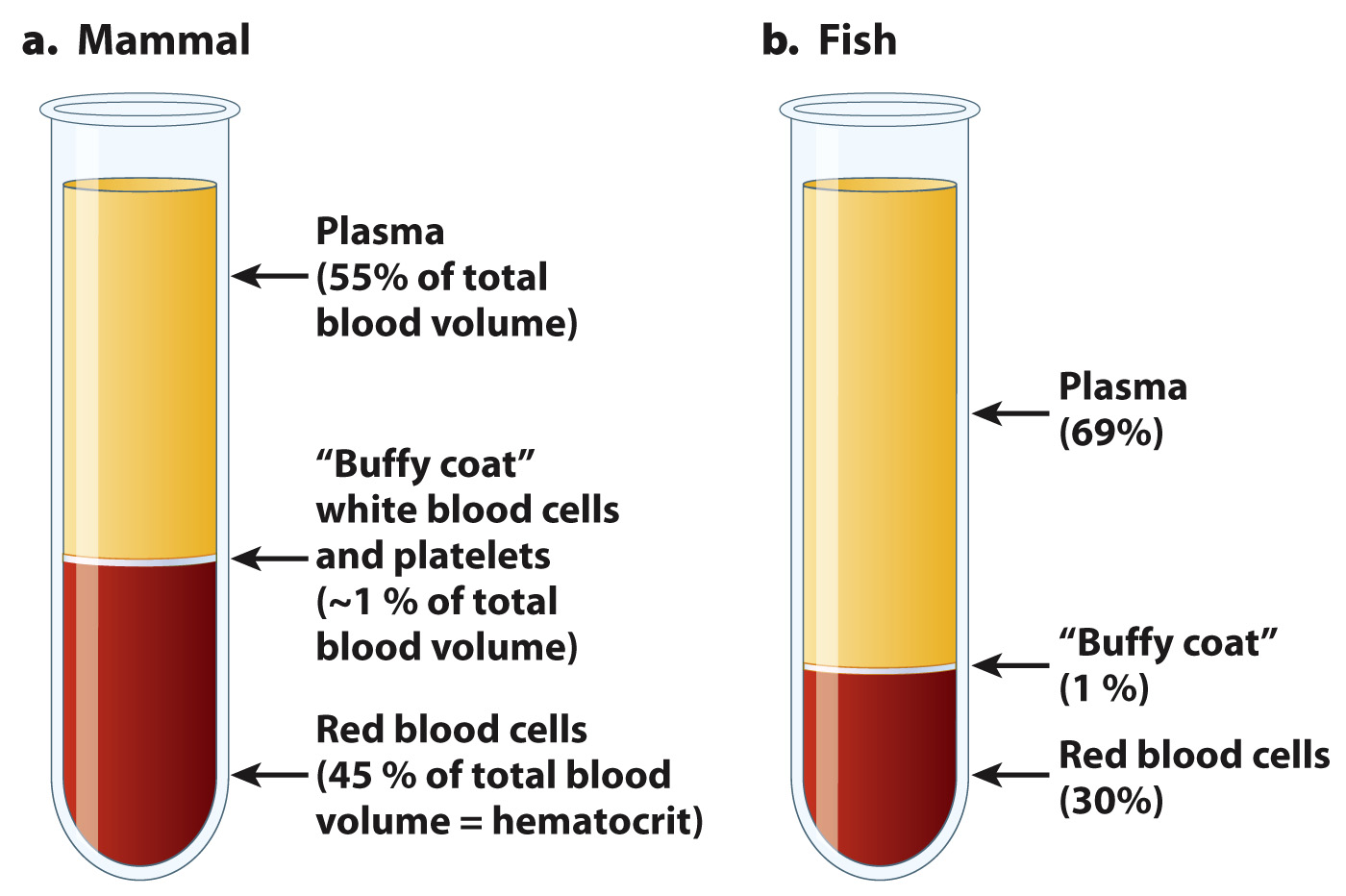
A much smaller fraction (~1%) of cells are white blood cells, which help to defend the body against pathogens. Their role is discussed in detail in Chapter 43. The remaining cells are platelets, which respond to damaged blood vessels by helping to form a clot to prevent blood loss.
When blood is spun in a centrifuge, the red blood cells, the densest component of blood, form a layer in the bottom of a centrifuge tube, and a thin “buffy coat” layer of white blood cells and platelets forms at the top of the cellular fraction. The remaining contents are the blood plasma and solutes, which rise to the top of the centrifuge tube (Fig. 39.11).
Blood plasma can hold only as much O2 or CO2 as can be dissolved in solution. How much O2 goes into solution at a given partial pressure is a measure of its solubility. Because O2 is about 30 times less soluble than CO2, only about 0.2 mL of O2 can be carried in 100 mL of blood. By binding O2 and removing it from solution, hemoglobin increases the amount of O2 in the blood a hundredfold. Because of its greater solubility, CO2 is transported in solution within the blood. Rather than remaining dissolved in solution as a gas, most of the CO2 (about 95%) is converted to carbonic acid, which dissociates to form bicarbonate ions (HCO3–) and protons (Chapter 6).
837
Whereas some invertebrate hemoglobins exist in solution in the hemolymph and others exist within cells carried by the hemolymph, all vertebrate hemoglobins are produced by and reside in red blood cells. Hemoglobin gives the cells and the blood their red appearance.