Myoglobin stores oxygen, enhancing delivery to muscle mitochondria.
Myoglobin is a specialized O2 carrier within the cells of vertebrate muscles. In contrast to hemoglobin, myoglobin is a monomer that contains only a single heme group. Because there are no interacting subunits, the O2 dissociation curve for myoglobin has a different shape from that of hemoglobin (Fig. 39.13b). In fact, over the physiological range of pO2, myoglobin has a greater affinity for O2 than hemoglobin does and binds O2 more tightly. As a result, hemoglobin releases O2 to exercising muscles. As the exercising muscles consume the available O2, the intracellular pO2 drops, and the myoglobin releases its bound O2 to the mitochondria.
839
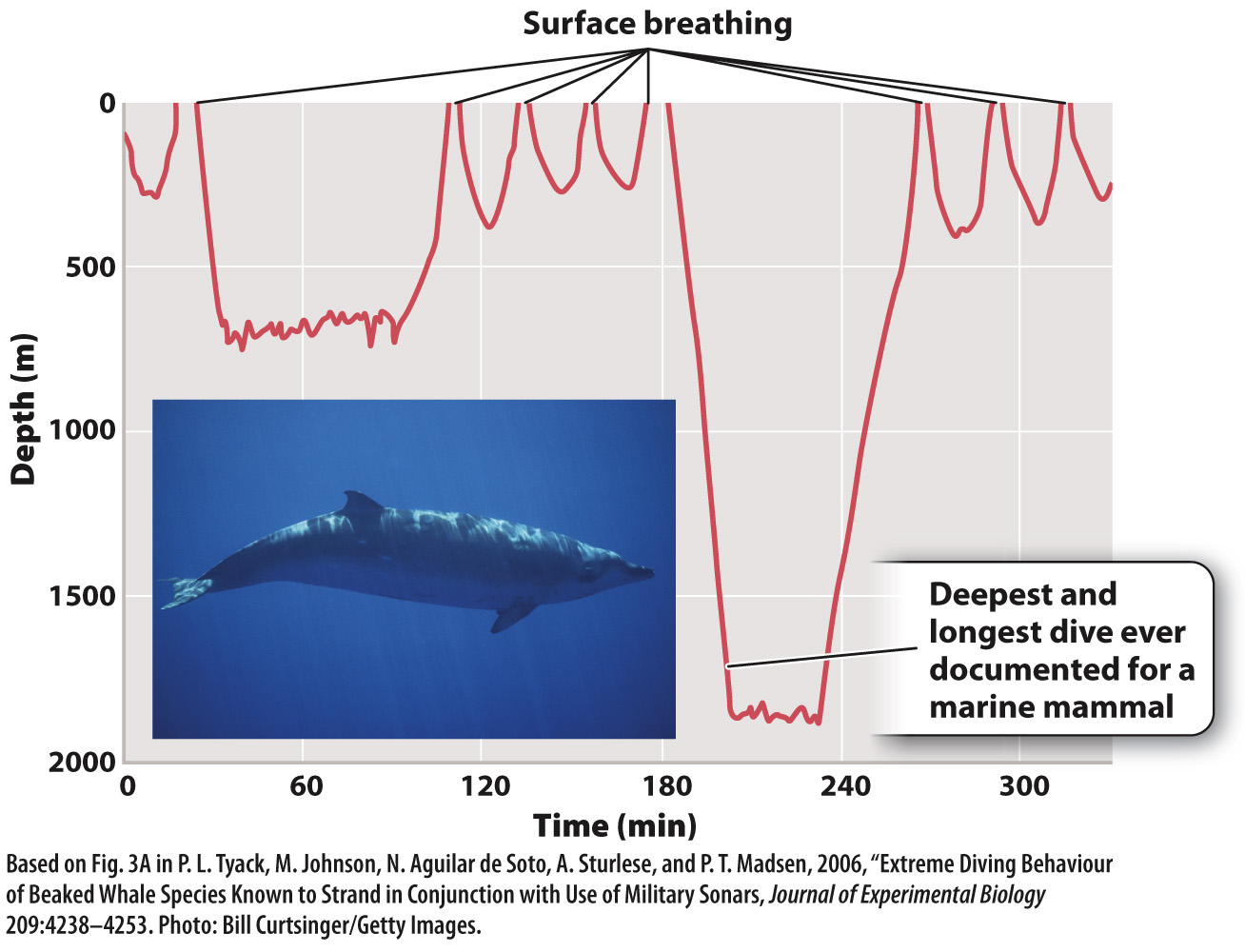
Red muscle cells that depend mainly on aerobic respiration to produce ATP (Chapter 37) store large amounts of myoglobin. The myoglobin in these cells can release O2 quickly at the onset of activity, before respiratory and circulatory systems have had time to increase the supply of O2. Diving marine mammals, such as whales and seals, carry large amounts of myoglobin within their muscles. The myoglobin loads up with O2 when the animals breathe at the surface before a dive. The O2 bound to the myoglobin is then used to supply ATP during the dive, when the animal cannot breathe. These marine mammals can stay under water for 30 minutes or longer and dive to considerable depths (Fig. 39.14).