A DNA strand consists of subunits called nucleotides.
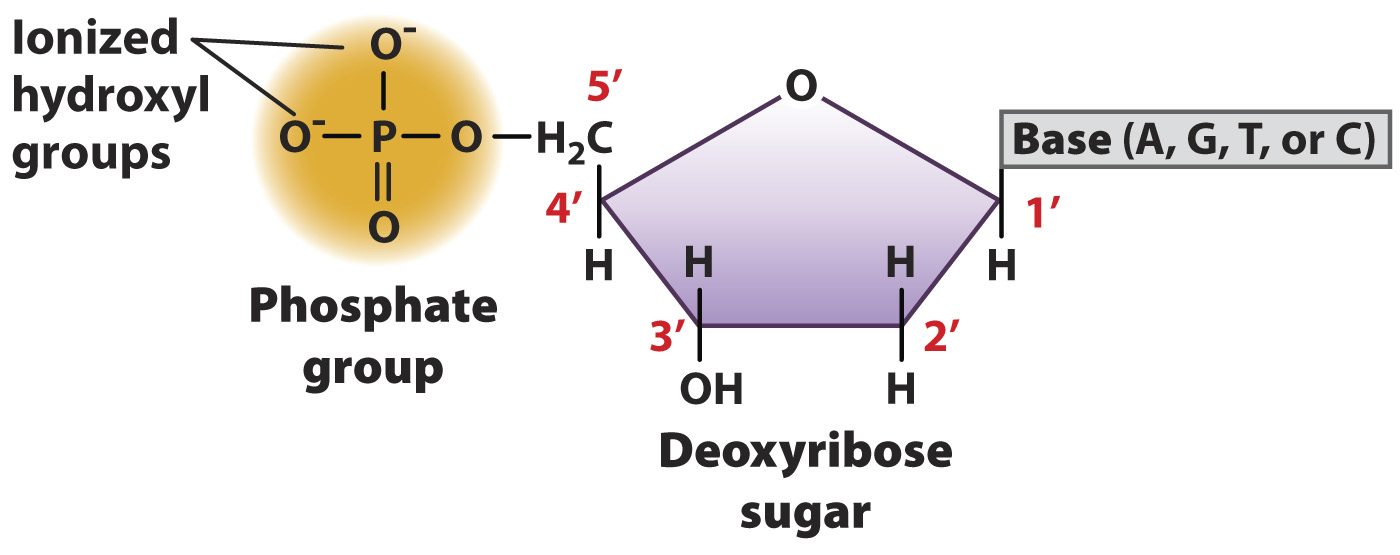
In the sixty years since the publication of Watson and Crick’s paper, we have all become familiar with the iconic double helix of DNA. The elegant shape of the twisting strands relies on the structure of DNA’s subunits, called nucleotides. As we saw in Chapter 2, nucleotides consist of three components: a 5-
DNA had been discovered 85 years before its three-
Note in Fig. 3.4 that the phosphate group attached to the 5′ carbon has negative charges on two of its oxygen atoms. These charges are present because at cellular pH (around 7), the free hydroxyl groups attached to the phosphorus atom are ionized by the loss of a proton, and hence are negatively charged. It is these negative charges that make DNA a mild acid, which you will recall from Chapter 2 is a molecule that tends to lose protons to the aqueous environment.
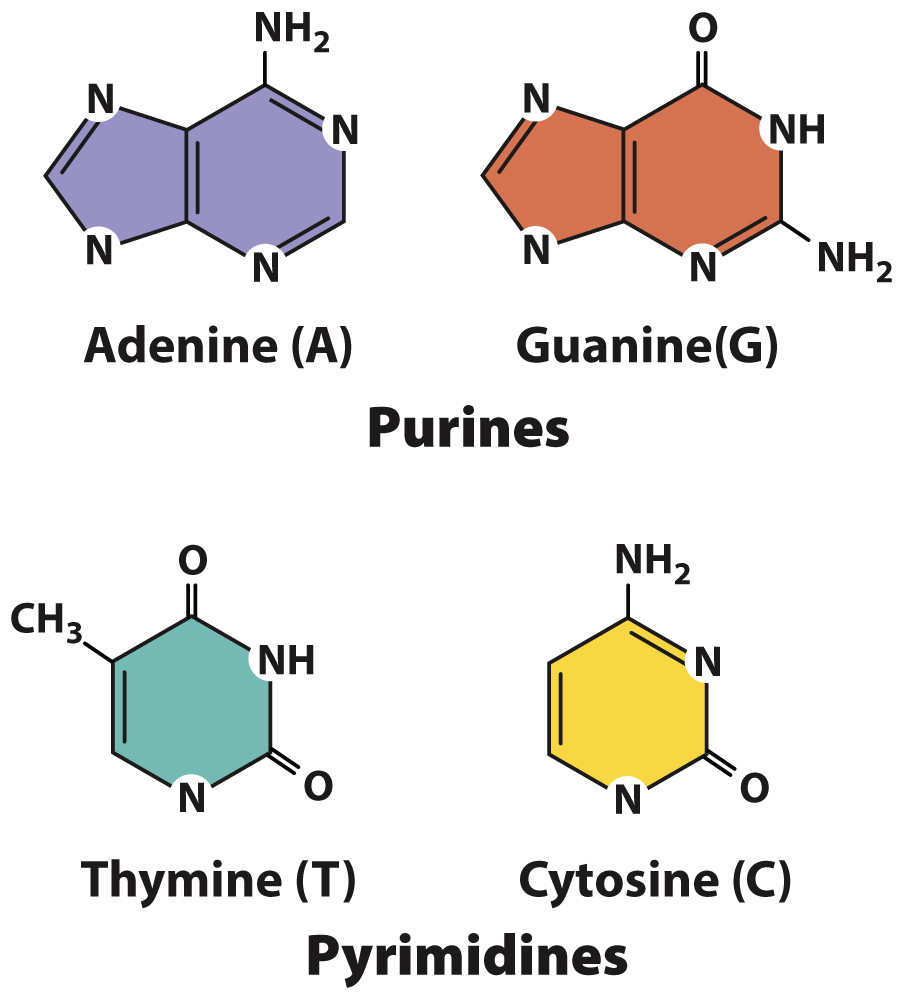
Each base is attached to the 1′ carbon of the sugar and projects above the sugar ring. A nucleotide normally contains one of four kinds of bases, denoted A, G, T, and C (Fig. 3.5). Two of the bases are double-
54
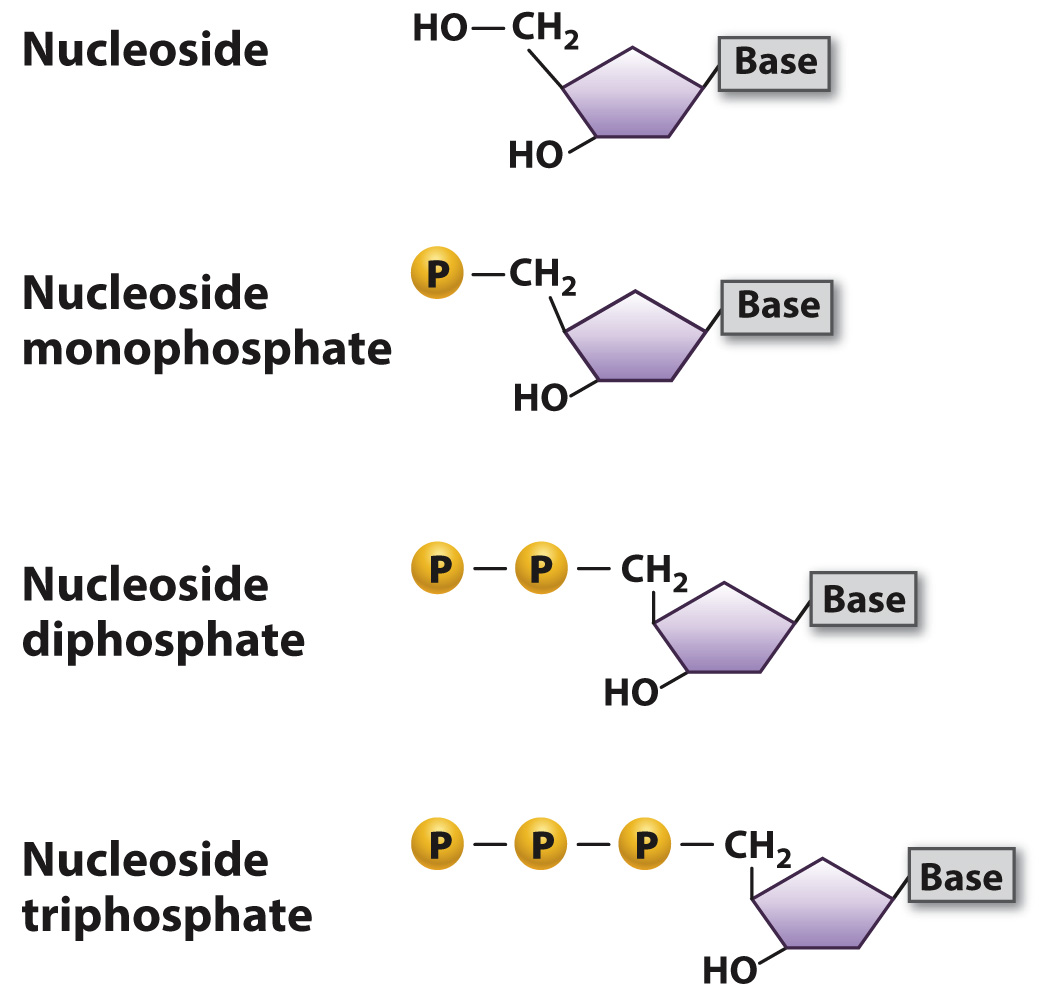
The combination of sugar and base is known as a nucleoside, which is shown in simplified form in Fig. 3.6. A nucleoside with one or more phosphate groups constitutes a nucleotide. More specifically, a nucleotide with one, two, or three phosphate groups is called a nucleoside monophosphate, diphosphate, or triphosphate, respectively (Fig. 3.6). The nucleoside triphosphates are particularly important because, as we will see later in this chapter, they are the molecules that are used to form nucleotide polymers: DNA and RNA. In addition, nucleoside triphosphates have other functions in the cell, notably as carriers of chemical energy in the form of ATP and GTP.