Osmosis governs the movement of water across cell membranes.
Water, like all molecules, moves by diffusion, or the random motion of molecules (Chapter 5). When there is a difference in water concentration between two regions, there is a net movement of water molecules from the region of higher water concentration to the region of lower water concentration. Water often contains other dissolved molecules, such as electrolytes, amino acids, and sugars, which are collectively called solutes. Because we typically describe solutions in terms of the concentration of dissolved solutes, it is sometimes easier to think about water moving from a region of lower solute concentration (higher water concentration) to a region of higher solute concentration (lower water concentration). Whether considered in terms of water concentration or solute concentration, the direction of net water movement is the same.
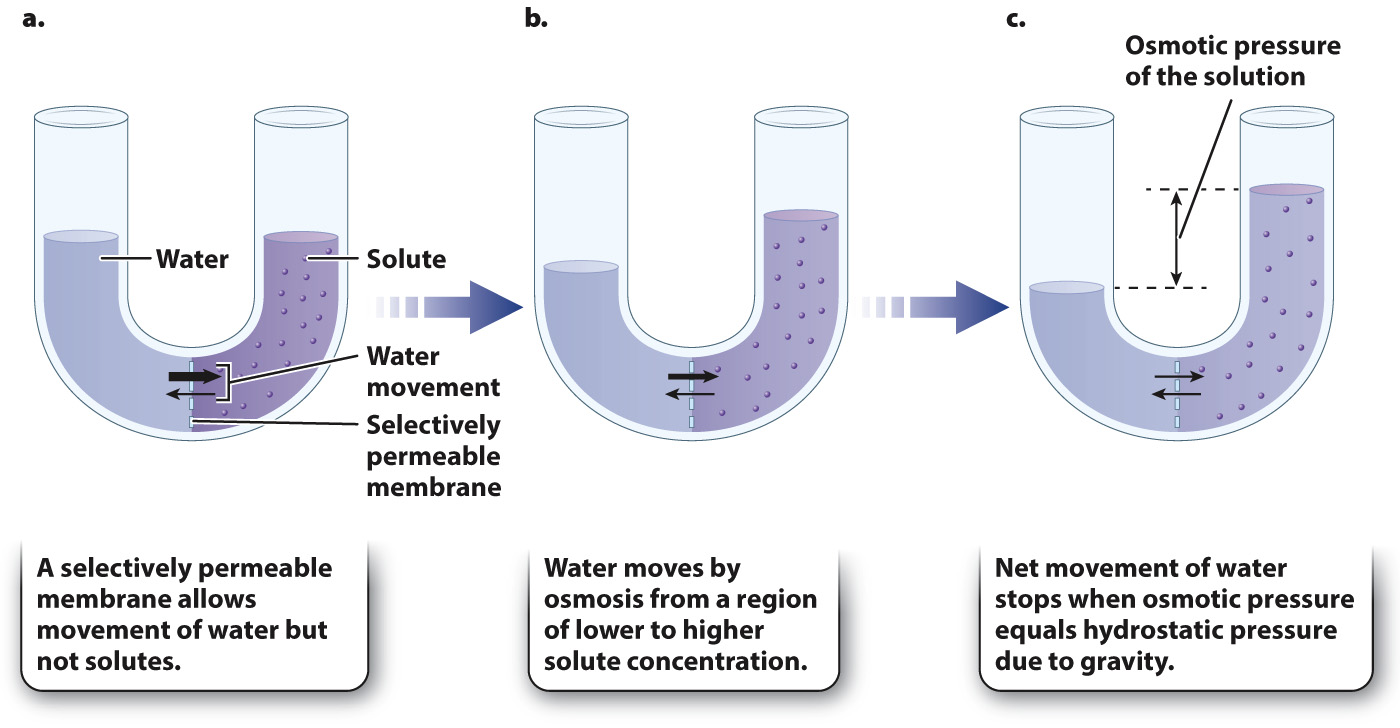
A barrier can sometimes separate two solutions. When a barrier allows water or solutes to diffuse freely, it is said to be permeable. When it blocks the diffusion of water or solutes entirely, it is impermeable. When it allows the movement of some molecules but not others, it is selectively permeable.
In organisms, the plasma membrane can serve as a barrier between solutions, as we saw in Chapter 5. The plasma membrane is a lipid bilayer with embedded proteins. It is selectively permeable: Water and smaller solutes can diffuse freely through the membrane, but larger solutes, like proteins, cannot. Water is able to move directly through lipid bilayers by diffusion, or through channels called aquaporins by facilitated diffusion.
The process by which water moves from regions of higher water concentration to lower water concentration across a selectively permeable membrane is called osmosis (Fig. 41.1). Consider a situation in which there is a difference in solute concentration on the two sides of a selectively permeable membrane that is freely permeable to water but impermeable to the solute (Fig. 41.1a). Water moves by osmosis from regions of higher water concentration (lower solute concentration) to regions of lower water concentration (higher solute concentration) (Fig. 41.1b).
Quick Check 1 What happens when a cell is placed in a hypotonic solution (one with a lower solute concentration than that of the cell)? What happens when a cell is placed in a hypertonic solution (one with a higher solute concentration than that of a cell)?
Quick Check 1 Answer
When a cell is placed in a hypotonic solution, water moves into the cell by osmosis and the cell swells or bursts. When a cell is placed in a hypertonic solution, water leaves the cell by osmosis and the cell shrinks.
The pressure needed to prevent water from moving across a selectively permeable membrane is referred to as osmotic pressure. Osmotic pressure is the tendency of water to move from one solution into another by osmosis. The higher the solute concentration, the higher the osmotic pressure of that solution (that is, there is a greater tendency for water to move into that solution). As water diffuses across a selectively permeable membrane into a region with a higher solute concentration (higher osmotic pressure), the hydrostatic pressure of the more concentrated solution increases. Hydrostatic pressure can result from gravity (if the solutions have different levels) or from the stiffness of the container walls. When osmotic pressure equals hydrostatic pressure, the net movement of water stops and equilibrium is reached (Fig. 41.1c). At equilibrium, water molecules still move across the membrane in both directions by diffusion, but there is no net movement of water molecules.
877
Whether water moves by osmosis into or out of a cell depends on the solute concentration of the inside of the cell relative to the outside. Similarly, whether water moves by osmosis into or out of an organism depends on solute concentration inside an animal relative to its environment, as well as the properties of the sheets of cells that make up the skin and other surfaces exposed to the environment.
Note that water movement is driven by differences in total solute concentration between the two sides of a cell membrane. That is, if a particular electrolyte such as potassium has a high concentration inside the cell and low concentration outside the cell, but another electrolyte, such as sodium, has exactly the opposite distribution, there will be no net water movement because the total concentrations of the solutes on the two sides of the membrane are the same.
Also note that water moves across selectively permeable membranes in response to concentration differences of any solute. That is, the solute can be an electrolyte, such as sodium or potassium, or another dissolved molecule, such as glucose or an amino acid or even waste, as long as the membrane is impermeable to it.
Quick Check 2 A solution of water and dissolved ions is separated by a selectively permeable membrane that allows the passage of water but not small ions. The concentration of sodium is higher on one side of the membrane than on the other side. However, there is no net movement of water across the membrane. How is this possible?
Quick Check 2 Answer
Osmosis depends on the difference in total solute concentration on the two sides of a membrane. There is no net movement of water molecules across a selectively permeable membrane with a high concentration of a particular solute on one side of a membrane if this concentration is matched by a similar concentration of some other solute on the other side of the membrane. For example, the concentration of sodium ions may be high on one side of a selectively permeable membrane and low on the other, whereas the distribution of potassium ions may be just the reverse, so the total solute concentration is the same on the two sides of the membrane. The result is no net water movement.