Nitrogen fertilizer transported to lakes and the sea causes eutrophication.
As discussed in Chapter 26, all organisms require nitrogen to synthesize proteins, nucleic acids, and other molecules. Although nitrogen is plentiful in the atmosphere as N2 gas, this form is not biologically available to animals and plants. Nitrogen-
Through the use of fertilizer and other activities, humans add about 140 million tons of fixed nitrogen to the biosphere each year, an amount comparable to that contributed by microbial nitrogen fixation. The majority of this fixed nitrogen is created industrially by reacting nitrogen gas and hydrogen in the presence of a catalyst to produce ammonia. Most commercially produced ammonia is further processed to nitrate, which is spread across fields as fertilizer to increase crop production. Success in feeding the world’s people owes much to fertilization with nitrates, but in fact, only about 10% of the nitrogen added to croplands ends up in food. Much of the nitrate fertilizer leaves fields as surface runoff and travels by rivers to lakes or the ocean. Denitrifying bacteria also use some of the added nitrate for respiration, returning N2 to the atmosphere—
In lakes and oceans, the fertilizing effects of agricultural runoff are finally realized, but in unintended ways. The added nutrients lead to a great increase in the populations of algae and cyanobacteria in a process called eutrophication. These algal and cyanobacterial masses eventually sink to the bottom, where heterotrophic organisms, mostly bacteria, feed on them, fueling high rates of aerobic respiration. The bacteria’s demand for oxygen can completely deplete O2 in bottom waters, with potentially catastrophic consequences for animal life on the seafloor or lakebed.
In the Gulf of Mexico, nutrients leached from croplands and transported down the Mississippi River result in an ominously named dead zone of oxygen-
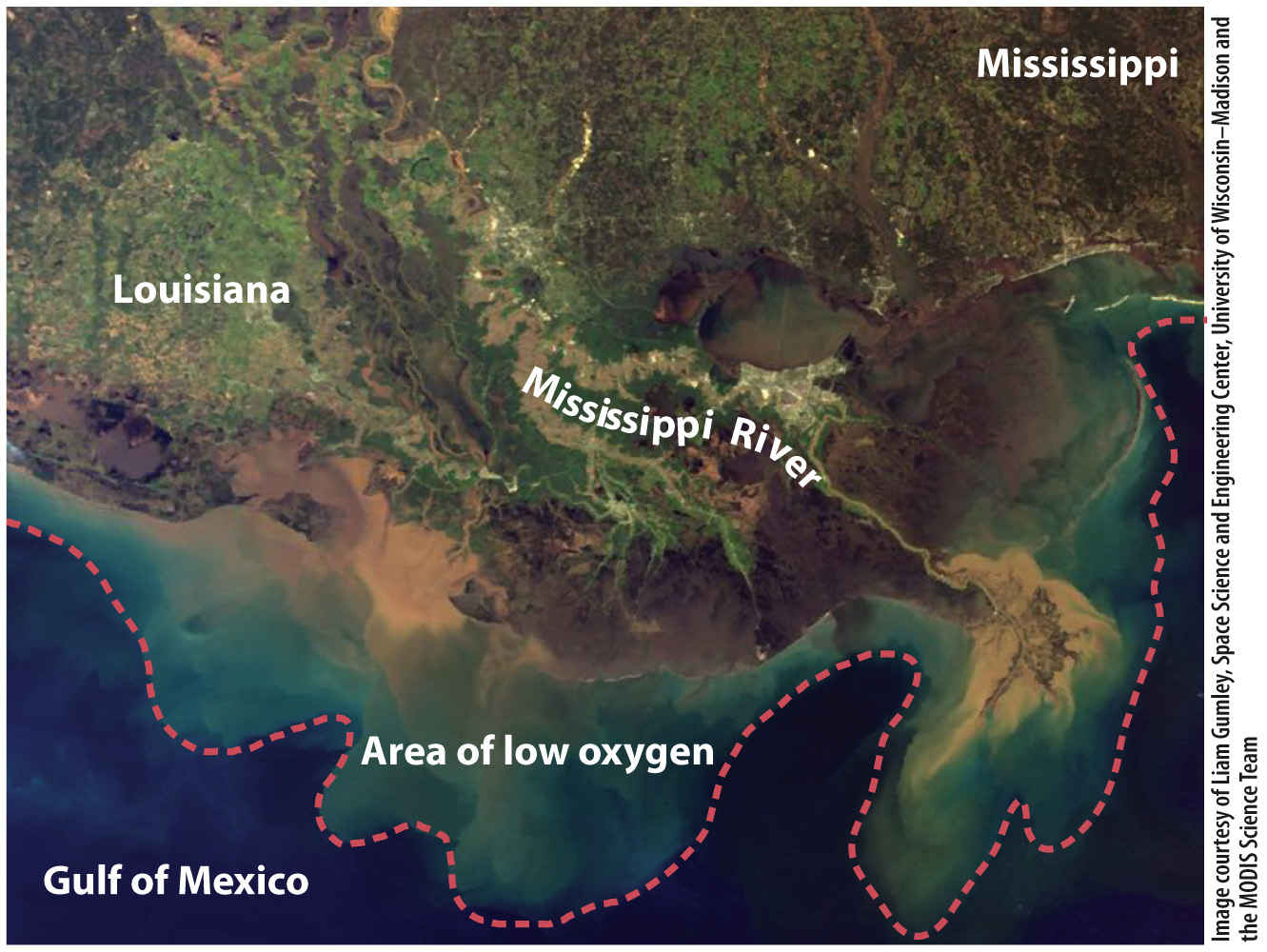
Nitrogen applied as fertilizer but spread by runoff and streamflow can affect the full range of ecological interactions discussed in Chapters 46 through 48. We benefit tremendously from increased crop production but pay a price in the eutrophication of lakes and oceans. We pay another price as well—