Case 8: How has global environmental change affected coral reefs around the world?
CASE 8 BIODIVERSITY HOTSPOTS: RAIN FORESTS AND CORAL REEFS

Increasing atmospheric CO2 is not just influencing life on land. It is also having an observable impact on organisms in the oceans, not least on coral reefs that provide hotspots of biological diversity in the marine realm. The Great Barrier Reef is visible from space, its wave-
1078
Corals are the principal architects of the Great Barrier and most other reefs (Chapter 44), and so coral biology is the key to understanding both the reef’s unusual diversity and its vulnerability in the face of climate change. Most reef corals gain nutrition from unicellular algae that live within their tissues. The algal symbionts in reef corals provide food for their host, in return receiving nutrients, a means of waste disposal, and a stable environment. It is the efficient cycling of carbon, nitrogen, and phosphorus between corals and their symbionts that underpins the biological richness of coral reefs. Reef corals accomplish another noteworthy feat: They make skeletons of calcium carbonate (CaCO3) that through time build the reef’s three-
The very processes that facilitate reef growth—

A greater long-
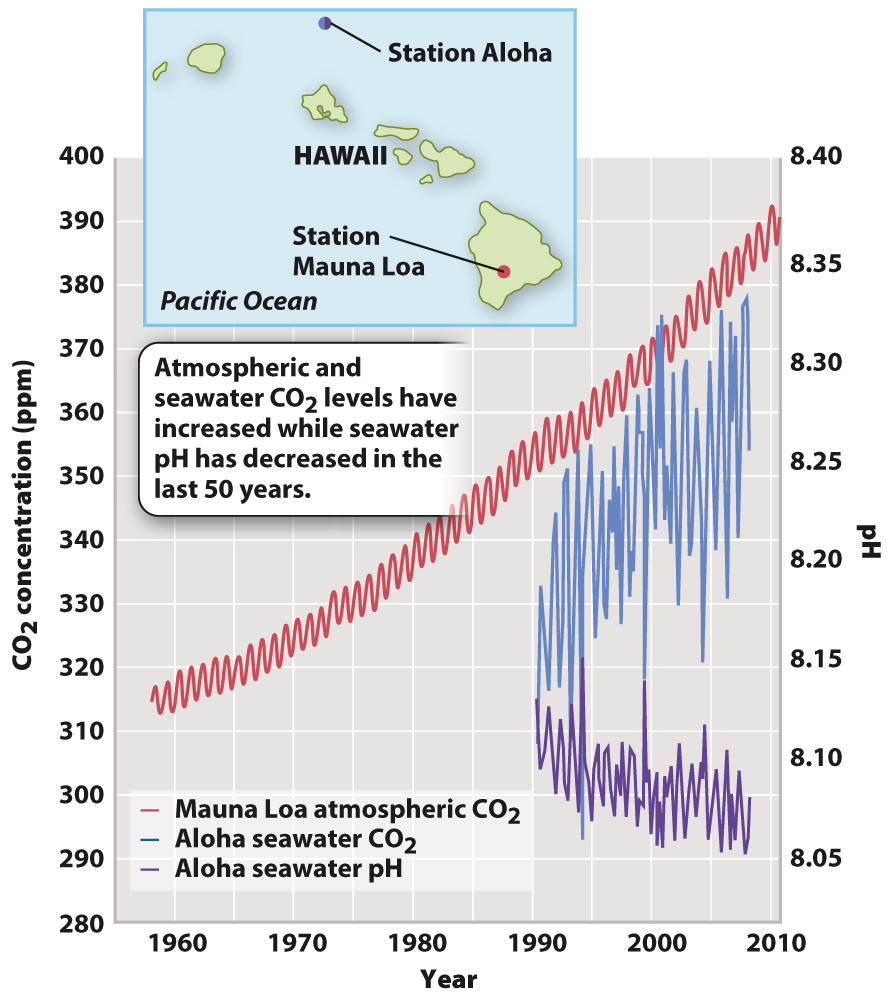
In the past 50 years, as the concentration of CO2 in the atmosphere has grown from about 315 ppm to about 400 ppm, the pH of surface oceans has correspondingly dropped by 0.1 unit (Fig. 49.13). That may not seem like a lot, but remember that the pH scale is logarithmic, and so the 0.1 unit drop represents an increase in hydrogen ions of about 30%. In turn, ocean acidification causes carbonate ions in seawater to decrease, making it more difficult for some marine algae and corals to build their CaCO3 skeletons (Fig. 49.14).
1079
1080
HOW DO WE KNOW?
FIG. 49.14
What is the effect of increased atmospheric CO2 and reduced ocean pH on skeleton formation in marine algae?
BACKGROUND It is well established that atmospheric CO2 levels are increasing, which in turn decreases the pH of ocean water. In the late 1990s, experiments showed that a decrease in pH affected the ability of some marine organisms to build skeletons made of calcium carbonate (CaCO3). The German biologist Ulf Riebesell and his colleagues carried out experiments to investigate whether ocean acidification affects algae called coccolithophorids, which account for the majority of carbonate precipitation in the open ocean.
HYPOTHESIS From the effects of decreased pH on CaCO3 skeleton formation in other marine organisms, Riebesell hypothesized that increasing CO2 would interfere with skeleton formation in coccolithophorids.
EXPERIMENT Riebesell and his colleagues studied two species of coccolithophorids, Emiliania huxleyi and Gephyrocapsa oceanica. They grew both species in the laboratory under conditions of increasing CO2, from pre-
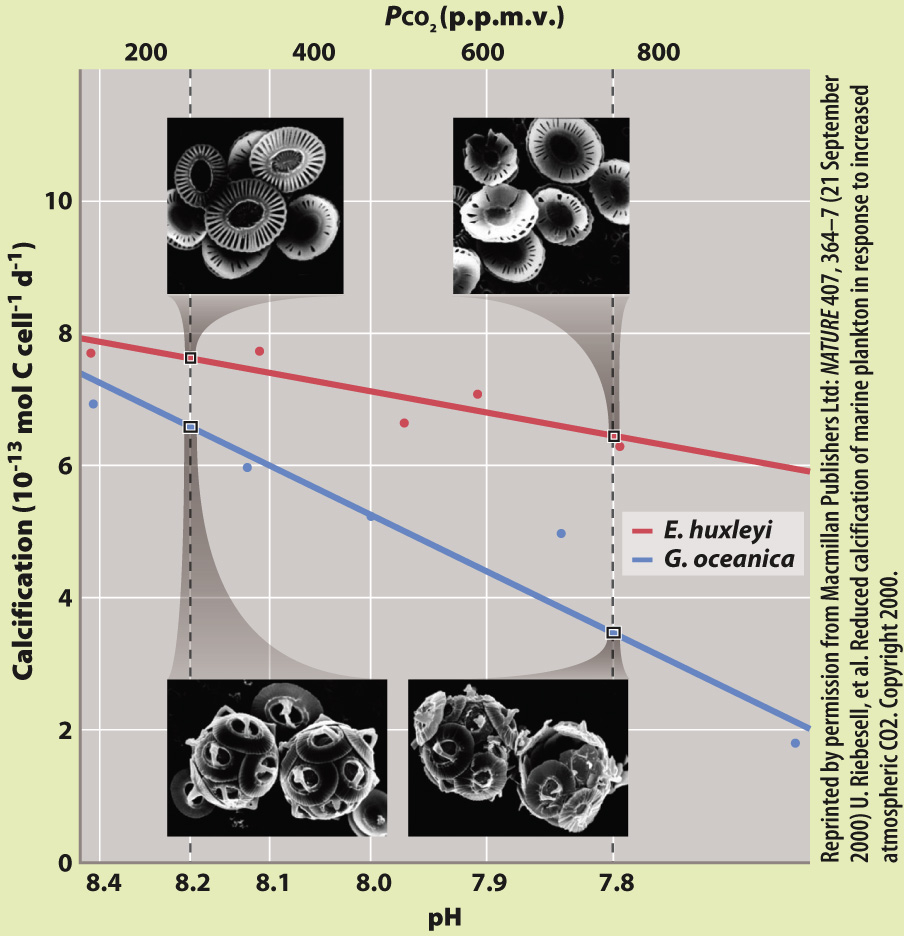
RESULTS With increasing CO2 and decreasing pH, they observed decreasing rates of calcification as well as deformed and incomplete coccolithophorids under the scanning electron microscope.
CONCLUSION The results support Riebesell’s hypothesis: Ocean acidification interferes with normal skeleton formation in marine plankton.
FOLLOW-
SOURCES Riebesell, U., et al. 2000. “Reduced Calcification of Marine Plankton in Response to Increased Atmospheric CO2.” Nature 407: 364–
Essentially, all attempts to model our environmental future predict that, by the end of this century, atmospheric CO2 will rise from its current level to more than 500 ppm, and changing pH in many parts of the ocean will limit the ability of corals to build skeletons. Some corals can survive this change. For example, experiments on two Mediterranean corals showed that at a pH level 0.8 units below that of present-